In 1963, a neurophysiologist named José Delgado demonstrated the effects of invasive electronic brain stimulation. He implanted electrodes into the brain of a highly aggressive bull, and then joined the animal in a bullring, armed with a hand-held radio transmitter to remotely activate the electrodes. When the bull began to charge, Delgado pushed a button on his transmitter, causing the bull to hold back.
Two years later, the experiment made front-page news in The New York Times, and the footage still makes for compelling viewing today. Delgado’s experiment marked the growing scientific interest in the use of invasive neurostimulation in the mid-20th century. Procedures that provided electrical stimulation to the brain via surgically implanted electrodes began to be used in the treatment of chronic pain, movement disorders such as Parkinson’s disease, epilepsy and psychiatric conditions. Toward those ends, researchers used the technology to stimulate (and thus, identify) malfunctioning areas of the brain. After impaired areas were identified through neurostimulation, invasive neurosurgery would eliminate the targeted regions in a technique known as lesioning.
Researchers of the era also wondered whether neurostimulation alone might be able to modify behaviour and emotional states. But early experiments were ethically dubious at best. Among the most infamous was an attempt to use neurostimulation to ‘cure’ an individual of homosexuality in 1972, a year before homosexuality was removed from the American Psychiatric Association’s Diagnostic and Statistical Manual of Mental Disorders (DSM). Here, the psychiatrist Robert Heath and his team at Tulane University electronically stimulated the septal region of the brain, associated with the experience of pleasure, then showed the male ‘patient’ films of heterosexual sex. As ‘treatment’ progressed, the patient went on to receive stimulation before having sex with a female prostitute hired by the researchers.
Such experiments (along with outcry against frontal lobotomy, which crudely severed connections in the prefrontal cortex, the centre of personality and thought) provoked a backlash. With the advent of powerful new drugs in psychiatry, the use of invasive neurostimulation came to be seen as archaic and cruel.
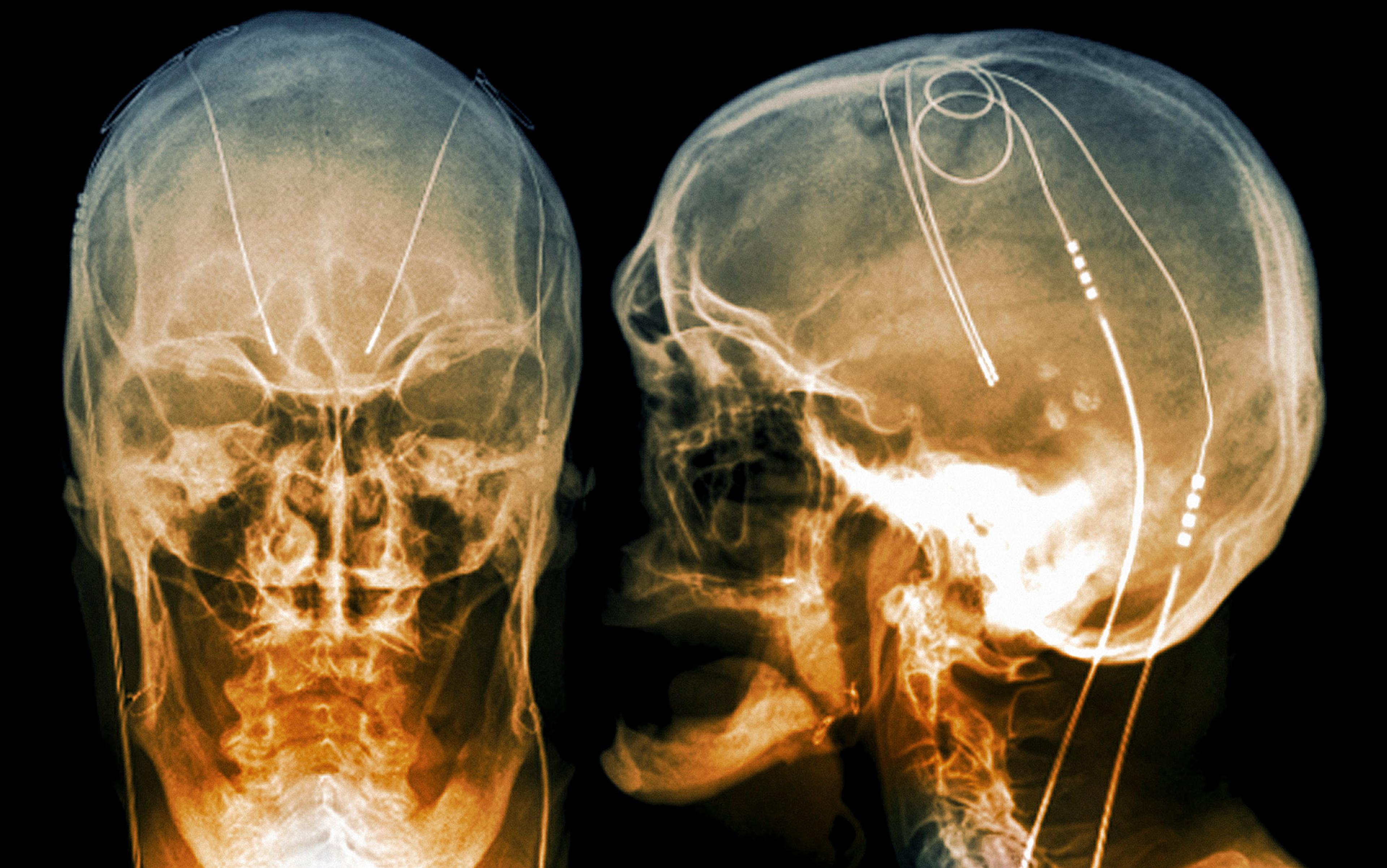
But today all that has changed. As the limitations and side-effects of psychopharmaceuticals became ever-more apparent, researchers began looking again at invasive neurostimulation – especially a safer, more precise version of the technology, called deep brain stimulation (DBS), which has been developed in the treatment of movement disorders in the decades since. The comparison between modern DBS and 1950s neurostimulation is stark. Early neurostimulation relied on an open method of surgery, involving the creation of a large opening in the skull, and an incision into the cortex to implant the electrodes, often leading to brain damage and infection. Inaccurate placement of electrodes was a serious risk.
By contrast, modern DBS surgery uses a ‘stereotactic’ frame that holds the patient’s head in place, and a computer-mapping system to generate a 3D representation of the patient’s brain. This can be used to identify neural structures, ensuring the precise placement of electrodes. In modern DBS, moreover, implanted electrodes are linked to a pacemaker-like device called an implanted pulse generator (IPG) under the collarbone. The IPG is programmed to send electrical impulses to the electrodes, personalising stimulation for that person alone.
The new therapy has revolutionised treatment of movement disorders for the sickest patients, and its safety and efficacy has been firmly established in clinical trials. In 2017, the US medical device company Medtronic announced that it had implanted its equipment in 150,000 patients worldwide. Just as Delgado halted the charge of a bull by pressing a button, so too can patients now reduce debilitating tremors and unintended movements by activating their DBS systems.
There is still a great deal of disagreement about whether stimulation reduces tremors by inhibiting, exciting or disrupting brain activity. But regardless of the underlying mechanism, the particular therapeutic effects depend on which area of the brain is being stimulated. Indeed, DBS to different areas of the brain is being investigated in a range of serious conditions where existing treatments remain ineffective. These include (among others) chronic pain, epilepsy, Alzheimer’s disease and disorders of consciousness such as coma. And work is underway to test DBS in psychiatric disease, with some promising, though preliminary, results for anorexia nervosa, obsessive-compulsive disorder (OCD) and depression.
The therapy is powerful, but complications and side-effects can still be severe. For one thing, we are talking about neurosurgery here. Although modern DBS is safer than earlier forms of invasive neurostimulation, it is still associated with significant risks. There are, of course, intraoperative and perioperative physical risks associated with the neurosurgical procedure itself, including (but not limited to) risks of haemorrhage, cortical infarctions and seizures. In the longer term, there are also risks associated with the maintenance of the DBS system, including (but not limited to) infection, lead-migration and skin erosion.
These issues alone mean that DBS should typically be considered only for those suffering greatly, and for whom all other treatments have failed. Suitable candidates for experimental DBS are thus often a highly vulnerable patient population.
A further risk that has gained notoriety recently is extreme personality change, with rare cases of previously balanced individuals becoming aggressive, apathetic or suicidal.
In a small number of cases, stimulation of the subthalamic nucleus (an area of the brain underneath the thalamus that modulates output from the basal ganglia, and which is the preferred target for DBS in Parkinson’s) has been associated with extreme shifts in mood and behaviour, with lives disrupted and derailed.
Affected patients might go on gambling binges and lose their life savings or land in catastrophic debt. In one reported case, a patient’s gambling became so pathological that he had to sell his house to finance his debts; his wife then asked for a divorce, and the patient attempted suicide.
Yet others may become hypersexual and manic. There have been reports of patients who have developed uncharacteristic sexual urges that they feel unable to control. One conservative, mature gentleman began to aggressively insist on sexual gratification every night following DBS treatment. Another report describes a woman who lost all inhibition under DBS, and who began to expose herself to male members of her family, demanding sex.
Under DBS, the patient became a devoted fan of Johnny Cash, banning other music he’d previously liked
Other times, changes can be more benign. One patient receiving stimulation to the nucleus accumbens, a part of the forebrain involved in reward and addiction, had not been that much of a music fan prior to DBS treatment. But, under stimulation, he unexpectedly became a devoted fan of the musician Johnny Cash, buying all of his CDs and DVDs, and banning other music that he had previously liked.
Supporters of DBS contend that dangerous personality changes, should they occur, can be programmed away or switched off. They say that the entire DBS system can (for some patients) be removed with a further neurosurgical procedure. In short, they contend that the damage is reversible. But claims about complete reversibility appear premature.
There are also further ethical dilemmas we face in treating patients with intractable disease. How should we decide which patients qualify for a DBS trial? How can we overcome obstacles to obtaining valid informed consent to the procedure? When is the right time to initiate largescale clinical trials, and what sort of trial design strikes the optimal balance between its scientific goals and the protection of patients? Of course, such questions pertain to other areas of medical research, yet they are particularly salient in DBS trials. As use of the technology has expanded, it raises the question: when are the side effects, including a new and potentially dangerous personality, worse than the disease?
Interview studies involving patient groups undergoing DBS for Parkinson’s and OCD provide insight into the mental effects and personality changes associated with stimulation, and the difficulties that patients and carers can experience as a result. Some patients tell interviewers that they have become a ‘different person’ following DBS. Others say that some of the unwanted side effects are ‘too much’ for them, and not a part of who they are. There are also examples where patients and spouses disagree if the individual has changed following treatment, and whether or not the change is beneficial. In one early patient interview study, the wife of a patient said she couldn’t cope with her husband wanting to live the life of a young man following his treatment, and that she wanted him to return to being more mellow.
In aggregate, the studies and interviews raise profound questions about the nature of identity, autonomy and our relationship to medical technology. But they must be placed in their proper context. DBS has been shown to lead to significant clinical benefits and improved quality of life for large numbers of movement-disorder patients, and it is still unclear how prevalent extreme, disruptive personality change has become. One obstacle to investigating the prevalence is that existing clinical assessment tools are not sufficiently fine-grained to capture all of these effects. While absence of evidence is evidence of absence, it is crucial that we not overhype these reports, just as it is crucial not to overhype the potential benefits of experimental DBS.
What’s more, although some of the changes observed in patients are often responsive to stimulation, it’s not always clear that DBS itself is directly responsible for inducing all of the changes observed in patients. A number of other factors could be responsible. Neurodegenerative conditions (such as Parkinson’s) often evince neuropsychiatric changes that can be made more apparent by ameliorating the patient’s impairment. Alternatively, changes reported by patients might plausibly arise as part of what has been termed the ‘burden of normality’, a concept that aims to capture the idea that individuals can experience adverse psychological, behavioural, affective and sociological effects when they receive effective treatment for a chronic condition. Changes to personality might also be attributable to other medications that have been associated with such effects, most notably levodopa (used in the treatment of Parkinson’s), and antidepressants such as paroxetine.
The fact that drugs are also associated with personality changes calls into question whether issues raised by DBS are unique. I contend that they are. Consider that the effects of drugs will wane over relatively short periods of time as the drug is metabolised. This means that patients might have frequent experience of what it is like to be ‘off’ medication, and their decision to take the next dose will typically be made when the effects of the drug have begun to diminish. In contrast, the effects of DBS do not wane in the same manner over the short term. A decision to reduce stimulation would thus have to be made when the patient is under the influence of stimulation, with all of its potential attendant effects on the individual’s decisionmaking processes. Moreover, some effects of stimulation can occur almost immediately.
DBS is a far more precise and adjustable method of altering brain activity than pharmacology. While pharmaceutical interventions will affect receptor activity in neurons across the brain, DBS can be used to hone in on much more specific neural targets (down to a cubic millimetre of brain tissue), and the parameters of stimulation can be altered to modulate activity in that target in various ways.
‘It is one thing to feel less anxious, but quite another to feel like a different person’
This degree of precision is important when we consider the effects of DBS on personality in the psychiatric context. While such changes are unintended in the treatment of movement disorders, the primary purpose of DBS in the psychiatric context might be to change personality, since the aim of treatment could be to alter aberrant emotional and motivational states. Of course, personality in the psychiatric context is hugely complex even in the absence of DBS, not least because of the so-called ‘ego-syntonic’ nature of some disorders, whereby patients identify themselves with their condition. A hyperconscientious or obsessive individual, for instance, might see that state of being as consistent with who they are, and not a deficit of personality at all.
It seems undeniable that in some cases patients have been harmed by their behavioural change. Yet, interview studies suggest that patients themselves do not always perceive ‘personality changes’ negatively. Is patient acceptance of any given personality change an important criterion for its benefit – even if the patient making the judgment is influenced by invasive stimulation that keeps the change alive?
Recent studies suggest that patients and their carers do, in fact, have a nuanced understanding of the moral implications of these changes, drawing on deeper understandings of their relationships, identities and essential selves. In an article in Plos One in 2017, a group of Dutch researchers interviewed 18 patients with OCD, asking them whether they had changed as a person. ‘For it is one thing to feel less anxious or more self-confident, but it is quite another matter to in addition feel like one has become a different person,’ the scientists write.
In the end, they found the questions about ‘personality change’ were too broad to capture what patients felt about their experience. The patients were changed, but often in ways that made them more like themselves.
‘I have become who I actually am, without OCD,’ said one.
‘Yes, that heavy load that I carried, that got lighter, that got less. I needed less time to pause at things, and think about things, so it took less time. Well, I was… yes, really changed 360 degrees,’ another explained.
The issues here are complex. On the one hand, it is the precision and adjustability of DBS that offers some basis for hoping that it can succeed where drugs have so far failed. On the other, the nature of the changes wrought, and their potentially longterm implications, means that we must take extreme care to get it right.
Rather than drawing general ethical conclusions for all personality changes following DBS across all of its applications, we need a moral framework that accounts for the variability of patient experience, case by case. The framework should help us to answer three questions: what does it mean for a person to change personality? How do we establish that this has happened? Why do such changes matter from a moral stance?
Perhaps the most significant moral challenge raised by reports of personality change following DBS is ensuring that future patients are as informed as possible about all the potential negative effects. A useful framework would invoke broad conceptions of identity and the self.
We might also consider whether changes are authentic to who the person ‘really is’. The experience of inauthentic behaviour, or of not ‘being yourself’, can simply feel bad. Even when it doesn’t, living authentically might plausibly be central to living a good life. But how can we capture the concept of authenticity? And if the person changes, would the new self still be capable of decisions reflecting that person’s core values and beliefs?
In early studies (including Heath’s use of stimulation to cure homosexuality), stimulation of neural targets associated with a pleasure were used to try to change sexual orientation. It is not difficult to imagine how the individuals in such experiments could come to feel deeply alienated from behaviours that they disvalued, but that they nonetheless had been conditioned to enjoy.
Similarly, in modern experimental applications of DBS in anorexia nervosa, some researchers have raised concerns that patients might be placed into a ‘psychological hell’ if stimulation increased patients’ eating behaviours without changing their distorted body image. The thought here is that simply inducing a motivation to eat in a patient for whom low body weight is of paramount importance might be experienced as completely alien to the patient and highly distressing. In contrast, if stimulation instead serves to reduce compulsive behaviours that the patient disowns, or to modify disordered emotional processing, then changes following treatment might more plausibly be understood as authentic.
As the technology progresses, other moral questions will emerge. For instance, how can we distinguish between changes that matter morally from those that do not?
The truth is that ‘reversibility’ is not simply a binary property of medical procedures but one of degree
Hovering over all these questions is the core issue of reversibility. One of the reasons that researchers initially became so interested in neurostimulation is that it represented a potentially reversible treatment, in contrast to brain-lesioning procedures such as the frontal lobotomy, depicted in books and films such as One Flew Over the Cuckoo’s Nest. But brain lesioning has come a long way since the frontal lobotomy – the procedures performed today also use stereotactic apparatus, making them far safer and more precise, and there are far more ethical protections afforded to patients. Yet even modern brain-lesioning procedures (such as anterior cingulotomies, a surgery that cuts part of the brain to treat depression, pain and OCD) have a significant downside: their effects, both adverse and beneficial, cannot be reversed.
Proponents point out that the adverse effects of DBS can often be circumvented by changing the stimulation parameters, or ceasing stimulation altogether. Naturally, this allows the patient to have far greater control and involvement in the treatment process. Moreover, the broadly reversible nature of DBS also means that it is an invaluable research tool in neurosurgery more broadly, allowing researchers to compare the therapeutic effects of modulating activity in the range of neural targets that might be implicated in a particular disorder.
However, as we learn more about the longterm effects of DBS, doubts about reversibility have emerged. Studies suggest that electrode insertion in DBS surgery and chronic stimulation can cause microlesioning and scarring in the brain, and there is also evidence to suggest that DBS leads to nonstimulation-dependent neural reorganisation, in which the brain is ‘rewired’, along with adverse psychiatric side-effects, and other serious complications.
This evidence undoubtedly suggests that it is a mistake to make the overly broad claim that DBS is wholly reversible. The truth is that ‘reversibility’ is not simply a binary property of medical procedures but one of degree. One must attend to both the reversible and irreversible effects of the procedure, and then compare their medical and moral significance.
Technological advancement might ultimately lead us to safer ground. Emerging research using focused ultrasound points towards a future of safer noninvasive forms of brain lesioning, while noninvasive forms of DBS are being investigated in mice. There are also a number of more developed initiatives that promise to improve the delivery of DBS, including closed-loop systems (which incorporate sensors that can detect brain activity and modulate stimulation accordingly) and miniaturised IPGs. As safer and more effective techniques emerge, new (and perhaps nontherapeutic) applications are likely to be considered.
But technological progress can also lead to new problems. One issue facing the future of DBS is the prospect of so-called ‘brainjacking’. Laurie Pycroft, a leading expert on DBS technology and cybersecurity at the University of Oxford, has explained, with his co-authors, that it might be possible for a hacker to access the IPGs incorporated into the DBS system, and exert control over its software settings. In addition to enabling access to private information stored on the IPG, brainjacking attacks could allow a third party to exert control over another person’s neurostimulation device. The prospect is alarming: even attackers who know nothing about their victim could still damage brain tissue by increasing stimulation beyond safe parameters, or exacerbate debilitating symptoms by ceasing the patient’s stimulation. Other times, attackers could seize control over the individual’s motivational and emotional states, with the ability to induce mania, hypersexual behaviour or a depressed state of mind.
Enhancing the safety and efficacy of DBS is an important goal that should guide future research. The prospect of brainjacking suggests that these efforts must be matched by efforts to ensure the cybersecurity of the devices involved. The safety, privacy, wellbeing and autonomy of a great many patients might depend on it.