Listen to this essay
23 minute listen
On the Hebridean island where I live, off the western coast of Scotland, windy days are smelly ones because large amounts of seaweed wash ashore, accumulating on the northern ends of beaches. For some, this might be an unremarkable occurrence, but here, on our small island, we see seaweed as a gift.
There are around 1,000 of us – crofters and office workers, incomers like me, and those who have stayed forever. Daily travel is dependent on the tides, the swells, and the weather at the ports that host the ferries. Limitations like these keep Earth’s cycles in continual focus. I like it this way. I lock my windows when a storm is coming, and I watch the waves. Afterward, I go and look for seaweed. In the winter, when storms are stronger, a man from the village next to mine drives his tractor down to the beach and drags the seaweed up to a higher spot. He creates a towering pile that smells like faeces. It feeds the island.
Here, we use seaweed as a fertiliser, because it is rich in elements that humans and plants both need for living, such as nitrogen and potassium. It also contains one particular substance that I have spent much of my life thinking about: phosphorus.

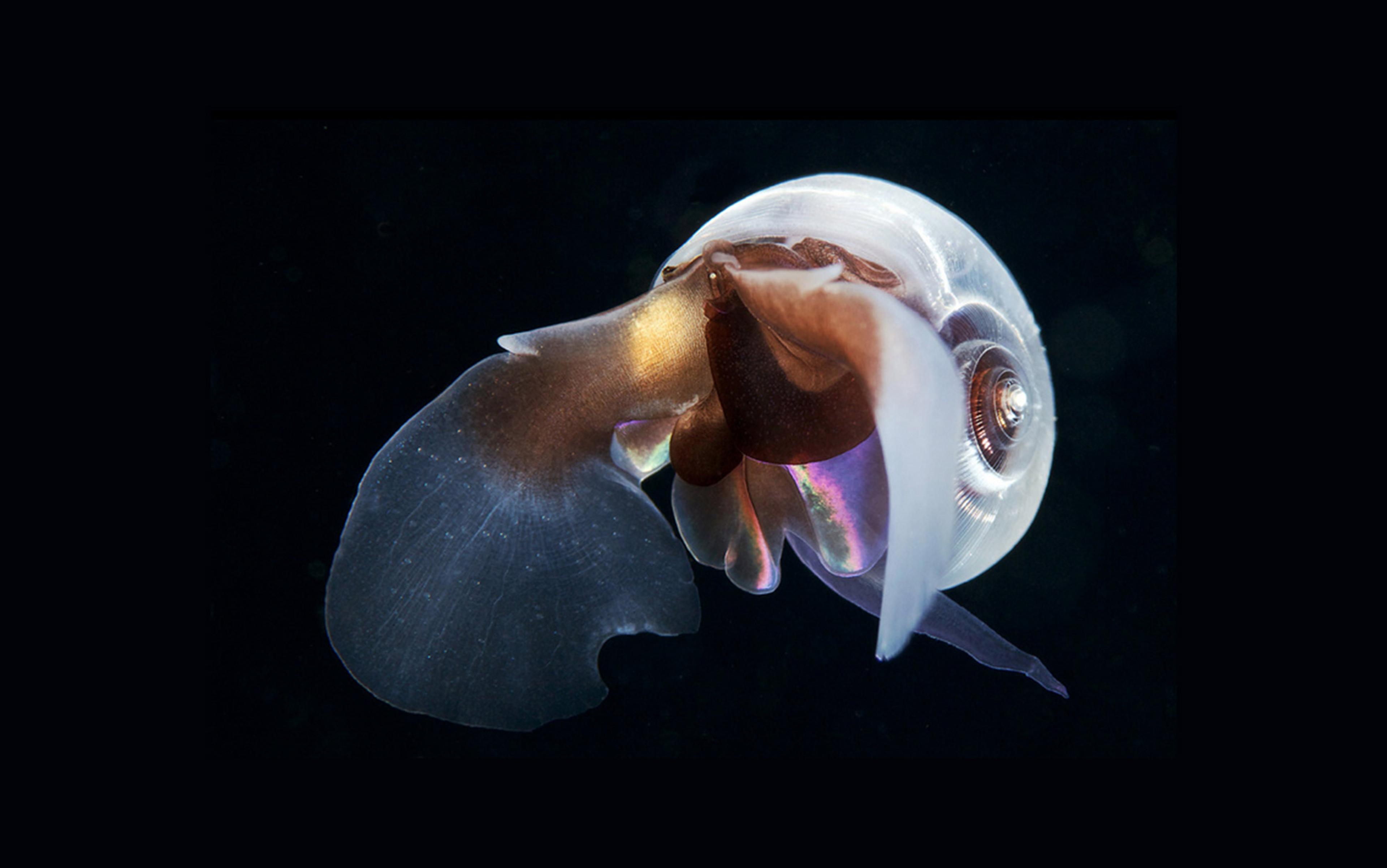
Seaweed on the shores of Benbecula, an island in the Outer Hebrides in Western Scotland. Courtesy Davide Gorla/Flickr
Stored in rock and organic material, phosphorus cycles slowly around Earth, through magma and mountains, down rivers, through waste and into oceans. Without it, there’d be no life – every living being needs it to grow. Unlike other mined materials, we all eat it. In the human body, it gives our cells energy, structure and identity, and it is particularly concentrated in our bones.
Rare enough to be notable, common enough to be necessary, phosphorus tracks the development of our planet. Over billions of years, as life and Earth evolved, phosphorus held the two together. Two hundred million years ago, when Pangaea broke apart, all those newly exposed continental edges leaked phosphorus into the oceans, leading to a biological boom. Something similar, though smaller, is happening today due to the rapid growth and uplift – and exposure – of the Himalayas. Trace amounts of phosphorus in mountains are enough to raise the biological productivity of the world.
Phosphorus fascinates me because it is a tangible expression of big ideas – a literal crystallisation of life on our planet. I am not the first observer to see worlds within this element. Phosphorus over the years has inspired bouts of marvelling and poetry, of reflection on our place within the world. In the 19th century, the theologian and geologist William Buckland found in phosphate ‘records of warfare, waged by successive generations of inhabitants of our planet on one another’. The naturalist Leonard Jenyns, some years later, saw in phosphate rock ‘the tracings of the fingers of God’.
This is a life-giving material that runs throughout Earth, a vibrant substance that is accessible to everyone. (Chemically isolated, it literally glows.) And yet humanity, on the whole, has turned it into a pollutant, creating life – and death – in the wrong places. In excess, it causes eutrophication, leading to algal blooms or sargassum outbreaks that suffocate other aquatic life. Our species’ relationship with this essential element has mirrored our own trajectory from living within nature’s cycles to imposing linear, extractive systems on the planet. By mining million-year-old phosphate and releasing it into the world’s waters, we are changing the biological composition, and even the geological structure, of the world. Understanding the phosphorus cycle means reckoning with our influence on Earth.
Across aeons, phosphorus limits life: it is the single element, on a global level, that determines the quantity of biomass in existence. That is because it is the rarest of those most necessary. Life may be constrained, in different places, by other elements or other circumstances, but within the context of the most extensive planetary measures, it is phosphorus that has been the great determinant.
The phosphorus cycle works like this: molecules, present in crust, make up trace amounts of all the rocks around us. They erode into rivers and feed life along the banks. Bits of rocks and sand in soils also contain phosphate crystals, which microscopic organisms break down. Much of the phosphorus that enters the biological world remains there. Beings die, they decompose, and then they are consumed again. But eventually, phosphorus returns to the ground, as organisms decay, lithify, and fossilise. Occasionally, across deep time, when phosphorus was locked away in rocks, there were moments when the world contained less life. But when continental edges were exposed to erosion, when mountain ranges rose up and weathered away, more phosphorus ran into the oceans, and productivity increased.
Just as phosphorus limits life, it also enables us to grow. It makes up cell membranes (phospholipids), enables energy storage (adenosine triphosphate), and gives structure to DNA (the sugar-phosphate backbone). It cannot be replaced and is, therefore, a required component of every new cell that is created.
The first element to be discovered through modern chemistry, it was named after the light it emitted in the lab
As a result, phosphorus has been a silent architect of human history. Early farmers relied on the phosphorus cycle. Civilisations were built where the soil was rich: along riverbeds that flooded, carrying phosphorus; in places where upwelling ocean waters produced phosphorus for the land nearby; on islands and near deltas where phosphorus collected. The Inca built their empire along coastline that matched the habitats of seabirds, which produced nutrient-dense guano. Ancient Egyptians developed intricate systems that trapped the phosphorus that rushed down the Nile; it had originated in the basalts of the Ethiopian Plateau.
When phosphorus was finally isolated, by an alchemist in 1669, it helped signal the beginning of modern chemistry, and it also marked the start of a new relationship between humans and Earth. It was the first element to be discovered through modern chemistry, and it was named after the light that it emitted in the lab. As humans better understood this substance, they became more deliberate in accumulating and using it on their crops. In those days, people often turned to skeletons. Dead soldiers were probably lifted at Waterloo, Egyptian mummies were ground into powder, the catacombs of Sicily were blitzed. Fossils, bones and fishmeal were shipped around the world for use as fertiliser. Distant islands, rich in certain excrements, were raided by whalers collecting nutrients on behalf of their respective flags. A global trade in bodies had begun.

The Alchemist Discovering Phosphorus (1771) by Joseph Wright of Derby. Photo courtesy Derby Museum and Art Gallery, England/Wikipedia
Over time, as farming became a more extractive industry, people discovered that some rocks contain particularly high concentrations of phosphate, the mineral that contains phosphorus. It was the beginning of the age of phosphate rock, which coincided with a time of European agricultural struggle. When nutrients were taken from the land, they were not replaced – land was a resource to be exploited, and people were viewed the same. The lands of Europe degraded first. As cities grew, good soil became more valuable. Some farmers took to grinding phosphate pebbles, found in pits or rivers, to supplement their soils, although the phosphate contained within those pebbles remained largely inert and inaccessible. Around 200 years ago, scientists found a way to make that phosphate available to the soil – to break its structure by treating it with acid – and geologists began to search for stores of buried phosphate rock.
That acid-treated phosphate rock was known as superphosphate, and as it rose in prominence so, too, did phosphate mining. From pits in England to islands in the Pacific to the growing industrial mines of the United States, China and Morocco, phosphate rock was excavated around the world. To reach the phosphate layer, the surface would be razed – chopped or burned – and the soil dug out. There were enough mines to make a significant change but few enough to ensure their value – as phosphorus remains rare, on a global scale.
Today, the phosphate industry mines fossil beds: places where life bloomed and was preserved, phosphorus along with it. Life from death, once again, but this time extracted from deep time. Over more than a century, billions of tonnes of phosphate have been lifted from those places – and, by doing so, humans have broken the phosphorus cycle.
Here is what else happened when people started mining phosphate rock: we stopped reusing animal manures and allowed them instead to run off into rivers. We stopped reusing human waste, leaving it in sewage in the sea. We stopped composting food waste. We began contending with the effects of that pollution – eutrophication of waters, the emergence of dead zones, where phosphorus fed algae that supplanted other organisms. We became reliant on bigger farms that needed mined fertilisers to function. We began to think that such a system is the only way to be.
Industrial agriculture relies on phosphate mining, and the mines produce, around the world, a quarter of a billion tonnes of phosphate every year. But this represents just a minuscule fraction of the total amount of phosphorus on Earth. Phosphorus is present everywhere, a circulatory system that runs around the world.
It is present in the structures of Earth; in the rocks that form geologic features; on the seafloor; in the seafloor; in the oceans; in algae and trees and animals and fungi and all the other living beings of this world. After all, living beings first concentrated it, leading to the deposits we mine today. At Earth’s beginning, when the planet was soupy, the elements were mixed together in solution. Phosphorus was, broadly speaking, evenly distributed. Early cells collected phosphate in order to exercise their core functions. As life grew more expansive, it used more phosphate.
A soil is not an easily replicable substance. It is complicated and alive
Every soil contains some amount of phosphate. Sand, whittled down from larger minerals, contains significant amounts of it. But that phosphorus is accessible only through the work of microorganisms: phosphate, on its own, cannot travel to the roots of plants. Fungi and bacteria break down the minerals in the soil, holding nutrients while living and then, in dying, converting them into an accessible organic form. Fungal networks transfer phosphate to plants. Without a healthy soil, the mineral remains inert.
When humans started mining phosphate rock, we created an agricultural system that devastated soils. Heavy machinery hardened the ground, pesticides killed microorganisms, and those that remained were starved by a lack of organic material (the stalks and leaves and half-digested grasses that make up manures and other natural fertilisers, rich in carbon, had been labelled, by the makers of synthetic fertiliser, inefficiencies). It seemed to work at first, but over time, as the soils deadened, the benefits have become less obvious.
The argument for this new system went like this: natural phosphate fertilisers are watery; they are heavy. Manure is difficult to move around: all that carbon, those stalks and grasses. And the places where animals and plants thrive are often different. (In England, for example, crops tend to grow in the east, animals in the wetter west.) To feed billions of people, you need a lightweight fertiliser applied with scientific precision.
That is the argument. The argument falls to pieces on the ground. A soil is not an easily replicable substance. It is complicated and alive. The crop plants grown through industrialised systems are, disproportionately, not those that provide the world’s nutrition. They are the cornfields grown for use in corn syrup. They are the sugar-cane plantations designed to produce cheap fuel. Phosphate fertilisers, made from mined material, are greasing the gears of the industrial state.
I began thinking about phosphorus when I was small, long before I ever learned about the periodic table or any chart of geological time. In the state of Virginia, where I grew up and where my grandmother lived, two hours away, on the edge of a city near the mouth of the Chesapeake Bay, I used to garden. In the little red toolshed behind her house, my grandmother kept a bag of dried bone powder. She applied it to her soil when she felt it needed phosphorus.
But this was not her only source of nutrients. She kept dried blood, rich in nitrogen; slaughterhouse waste sold as fertiliser. She had potash, containing potassium. She tended compost piles in a quiet corner of her garden, and this was how her plants continued to thrive.
In a century of mining, 80 per cent of the country’s surface area was stripped
I was entranced by this cyclicality. We were eating from the garden and feeding it all at once. The food scraps that she put into her compost bins transfigured into soil, seemingly by magic. When we opened a door at the bottom of each bin, rich soil would spill out. Those bins provided to me, even as a child, the reassuring notion that what goes out will ultimately return.
Nearby, the Chesapeake Bay was clogged with algae fed by phosphorus runoff. We learned, in school, that fertiliser and sewage enabled the blooms, which then decomposed, consuming oxygen and suffocating life. I thought about my grandmother’s garden – how assiduously she protected her soil, how opposed she was to losing any of her nutrients. If those nutrients had flowed out of her garden, they would have transformed from necessary to harmful, creating too much life in unwanted places. The depleted soil they left behind would have been less fertile. My grandmother built her soil up for decades, constructing berms and buffers to prevent the loss of nutrients through her fence.

Roughly 80 per cent of Nauru has been devastated by strip mining, as seen in this satellite image from 2002. Photo courtesy the US Department of Energy Atmospheric Radiation Measurement user facility
Later, I thought more about the origins of phosphorus. I went to Nauru, a Pacific island-state that had been mined for phosphate rock, but where deposits had in the ensuing years largely run out. In a century of mining, 80 per cent of the country’s surface area was stripped. I spent a month there. The mines had been transformed into refugee encampments. Within those spaces, in a programme sponsored by the Australian government, thousands of people from around the world had been held against their will. Moving through a topography of camps and ruins, I was struck by the emptiness of the land where phosphate used to be.
Now, when I think about phosphorus, I think about time. In the Pacific, where Nauru flourished, the movement of time is viewed as a spiral. Cycles layer upon one another as Earth continues to spin. My whole worldview is cycles – movement and connection. I see nature in terms of phosphorus, its local circuits entwined with more expansive ones. Each of our own individual actions will affect a phosphorus cycle, if not, in immediately obvious ways, the global one. That makes phosphorus a unique environmental problem. Every individual response makes a discernible difference. You can buy an electric car, operating on blind faith that your small contribution helps the climate. But if you start composting, you will quickly see your soil change.
I cannot help seeing, within these broken cycles, opportunity. We are provided with vast amounts of nutrients, with biological systems that nurture the soil indefinitely. It is beneficial for many reasons to recycle phosphorus, through age-old mechanisms, back into the ground. More life in the soil leads to more nutrients in food. It makes the soil more resilient in the face of disaster. It produces fertiliser without the need for outside inputs: there is generally enough phosphorus present in the soil – mineralised, inaccessible – to feed crop plants for many decades, provided there are microorganisms ready to digest it.
When land is concentrated in the hands of a few people, it produces food less efficiently
Despite this opportunity – this plentiful array of nutrients around us – we are told to rely on certain companies to give us phosphorus that has been mined, packaged and sold for profit. We are told this is a material accessible only through extractive expertise. This is the message of powerful institutions – of mining companies, agricultural corporations, billionaire philanthropists, and governments – that absent industrial agriculture, some large portion of the world would not be able to eat. Left unmentioned in those admonitions are the copious government grants propping up the fertiliser industry, the subsidies that stand between corporate agriculture and financial ruin: through these payments, the state keeps the phosphate industry afloat.
The push for synthetic fertilisers has always been connected to a certain lust for power. In 1842, when superphosphate got its start in England, wealthy landowners were beating back the poor. They faced a choice: raise yields through technology or through the redistribution of land. They chose the technological option, and phosphate mining began. A century later, the US government opposed new land reform movements around the world – in Mexico, India, the Philippines. The Green Revolution, as it came to be called, was designed to counter Red Revolutions, defending landowners and preventing mass unrest. When land is concentrated in the hands of a few people, it produces food less efficiently than when it benefits from local management. An outsider might find his farmland dying; he purchases a phosphate powder. A local, who knows the nature of the land, will head down to the seaweed pile and scoop.
The costs of mining phosphate and then wasting it are immense. We have created an expensive, unreliable and dangerous system of production. The most serious consequences are clear today. They are social and political: the surge of the upper class and the decline of farmers. They are medical: the rise of chronic illnesses as the quality of our food declines. When they are ecological, they are dramatic, as when our waterways are stuffed with algae and we cannot fish.
Across deep time, phosphate deposits do not form without the work of living beings. In phosphate rock, we find a record of Earth’s progress. Its most dramatic moments are preserved in stone.
What humans are doing, in mining and in moving phosphorus around on such enormous scales, is also geological: we are creating new layers. By removing ancient deposits and shifting them around, causing excess phosphate to drain into the ocean, we are leaving imprints that will long be visible.
Into the ocean we send our phosphate wastes, and they cycle for millennia
Eventually, though, Earth’s cycles will rebalance – it will just take millions of years. The anthropogenic layers that form on the ocean floor will subduct back under Earth’s crust – or else push up out of the sea and become continents. Those new layers are where trace amounts of phosphate come from, the molecules that rush down rivers, fertilising fields. This is an endless process. When we eat phosphorus and fertilise plants with it, we are still – despite our many perturbations – just participants.
The ocean is the conduit for that undiminished cycle. It is the sink, the place where phosphate goes to wait, where rock begins to form. Into the ocean we send our phosphate wastes, and they cycle for millennia. Areas of upwelling are formed of concentrated phosphorus, and they maintain life on our planet. And all along the ocean’s edge, we encounter little pockets of fertility: our connection to Earth. A storm occurs; it washes nutrients ashore; and they feed us.
The phosphorus in the seaweed on my island home probably entered the ocean thousands of years ago. It might have circulated during the time of Jesus, the Crusades, the birth of nation-states. Some of it might be new – added during the revolutions that shaped modern agriculture. Humans, after all, have doubled the flow of phosphorus into the oceans. The wastes that we are adding now will not emerge for years. The phosphorus in the ocean settles, churns, then exits. The storm abates. The crofter takes his tractor and scoops it up.

Gathering seaweed to fertilise the land. Photograph by Margaret Faye Shaw, c1932. Courtesy The National Trust for Scotland
The seaweed pile in my community is open for anyone to use. It builds up through the cold months of the year, and then it goes away as everyone prepares for planting. There was once a flag, long ago, that alerted other villages to surplus: anyone could take what they needed. Today that invitation is implicit. By summertime, the pile is gone.
It is a good deal easier to head down to the beach and shovel seaweed than it is to prospect, mine and manufacture phosphate fertiliser, and to deal with the wastes created by that process, some of them radioactive, all of them expensive to manage and clean. Seaweed is straightforward, and it is free. But it works only because the farms here are not massive, and that is the case only because the ownership of land has been restricted. Farms cannot be bought and merged together ad infinitum. They cannot be turned into factories, as farms have become in many other places, including in Virginia, where I first learned to fertilise the land. Here, we all profit from the output of the oceans, and we work together to grow our food. The crofter who piles seaweed does not charge us for his service; I think of him as a kind of natural phosphate miner. His is a social solution to a pressing social problem, egalitarian, true.
The old static caravan I live in shakes when the winds are powerful. Cabinets open and close autonomously, pantry items fall onto the floor. The roof has tears in it; water leaks onto the bed. Outside, sea spray pelts the windows. Droplets enter through the keyhole in the door. Everything outside is changing – the world is breathing. When the air calms, I go down to the shore. The island has returned to normal. The sea is low. The high-water mark is easy to find because the waves have lined up seaweed at their furthest reaches. Slimy, smelly, creepy. The phosphorus of the deep has come.