At the Woods Hole Oceanographic Institution on Cape Cod, Massachusetts, snowdrifts piled up outside shuttered T-shirt shops, and wind and whitecaps lashed vessels tethered to empty piers in the harbour. The flood of sun-tanned tourists and research students that descends on this place in summer was still months away. The only visitor was a winter storm that hung over the coast, making travel in and out of the cedar-shingled town impossible. In a research building downtown, at the end of a dimly lit hallway, Peter Wiebe sat with a stack of yellowed composition notebooks, reliving a lifetime spent on the ocean. Wiebe, a grizzled scientist emeritus, is transcribing his research cruise logs, which go back to 1962. His handwritten notes archive a half-century of twilit cruises in the Antarctic and languorous equatorial days surrounded by marine life.
‘It’s quite clear to me things are changing,’ he told me, after I asked him to think back on his decades on the ocean. ‘As a graduate student on one cruise, my logs talk about a hammerhead and two whitetips following the ship the whole time. On other cruises, we would fish for mahimahi and tuna, and occasionally catch a shark. Now we hardly ever see any big fish or sharks at all.’
Indeed, in oceanography, the big story over the past half century – the span of Wiebe’s career – has been the wholesale removal of the seas’ top predators through overfishing. But the story of the oceans for the coming century may be a revolution that starts from the bottom of the food chain, not the top.
‘I won’t be around to see it,’ Wiebe told me. ‘I wish I were.’
Plankton (taken from the Greek word for wanderer) are the plants, animals and microbes that are unable to overcome the influence of ocean currents, either because they’re too small, like bacteria, or because, as in the case of the indifferent jellyfish, they can’t be bothered. Wiebe’s speciality is zooplankton, the kaleidoscopic, translucent animal world in miniature, much of which feeds on even smaller photosynthetic life called phytoplankton. To make the jump from photosynthesis to fish, birds and whales, you have to go through zooplankton first.
Wiebe is part of a body of researchers worldwide working feverishly to find out how these grazers will be affected by an increasingly unfamiliar ocean, an ocean that absorbs 300,000 Hiroshimas of excess heat every day, and whose surface waters have already become 30 per cent more acidic since the dawn of the Industrial Revolution.
‘When I first started, the idea that you could actually change the pH of the ocean just wasn’t there – no one expected us to be able to do it,’ Wiebe told me. ‘Certainly, no one expected us to be able to do it at the pace we’re doing it, at a pace that far surpasses anything natural that has ever happened.’
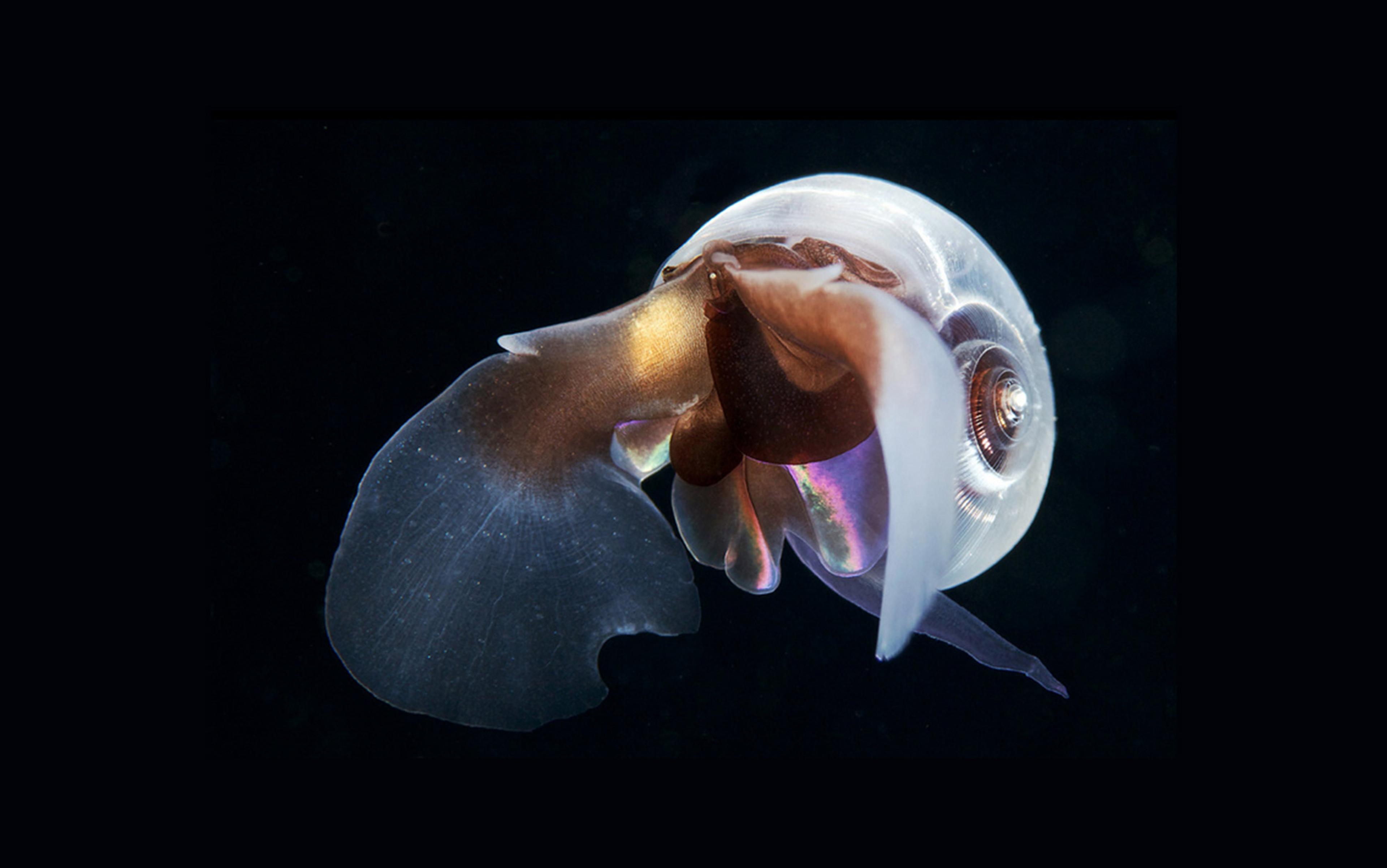
Nowhere on Earth are these changes more apparent than at the poles. Every year, in the Southern Ocean, swirling blue-green eddies of phytoplankton pulse with the seasons. These hurricanes of life are vast enough to be visible from space, but invisible in a handful of water. Pteropods – tiny translucent snails that long ago left the ocean bottom for a life of fluttering through the sea on winged feet – can mow huge patches of these carbon-rich blooms in a day. Along with other zooplankton such as krill, they drive the so-called biological carbon pump.
When zooplankton such as pteropods feast on the bloom, some of it sinks to the deep in a marine snowdrift of poop, half-eaten meals and other webs of protein that gather organic bits and pieces as they fall to the ocean floor. With 300 million tons of carbon shuttled to the deep every year in this manner, it’s a blizzard that can accumulate over time. The White Cliffs of Dover are one such edifice of plankton. And pteropods themselves, which sink like stones when they’re not beating their gossamer wings, can pile up on the sea floor by the billions, forming carbon-rich oozes.
However, just as important as their role in shuttling carbon to the deep is their place in the marine foodweb. Pteropods feed whales, fish and seabirds alike. They are the crucial second step in the transformative process that coverts sunlight into whales.
If they are esoteric to Westerners, pteropods are a bona fide cultural phenomenon elsewhere. In Japan, shell-less pteropods known as sea angels that prey exclusively on sea butterflies (their shelled relatives) frequently wash ashore, borne on currents from the arctic. The Japanese are obsessed with these visitors. Pteropods have inspired two Pokemon characters, a pteropod Hello Kitty, and tiny pteropod figurines that Sapporo occasionally packages along with its beer.
The roiling drama of the planktonic world is a theatre of ambush predators, hermaphrodites and mucus-hurling cannibals
Researchers are also doing their best to glamorise pteropods, in an attempt to garner public attention and funding. They are trying to rebrand pteropods and their ilk as ‘charismatic microfauna’ and with good reason. The roiling drama of the planktonic world is wilder than any savannah or jungle. It is a theatre of ambush predators, hermaphrodites and mucus-hurling cannibals.
If the film March of the Penguins (2005) was marketed on the premise that traditional family values prevail in the animal kingdom, the life cycle of pteropods would seem to represent a rebuttal. Male pteropods mate with each other, and then switch genders while holding onto the sperm, which they use to fertilise themselves. To feed, they cast out giant webs of slime that occasionally ensnare their fellow pteropods, whose innards they promptly suck out. The tiny snails have a softer side as well, with some species tenderly rearing their babies inside their shells. When they’re frightened, they nervously retract their wings, and plummet to the depths. It’s a sound strategy in a world where predators attack from all directions in space, with all manner of sci-fi appendages, including bioluminescent snares.
When they aren’t being hunted, much of the pteropods’ lives are spent dancing in the water column in a lilting flutter. The animals are hypnotising to watch, but it isn’t their ghostly beauty that attracts researchers. It is their alarming vulnerability. In the icy waters of the Antarctic, they are already beginning to dissolve.
In 2008, during an Antarctic research cruise north of the old whaling redoubt of South Georgia Island, the marine biologist Nina Bednaršek began to pull up net tows of the tiny marine snails. As expected, the nets were lined with pteropods, but something was off: their delicate calcium carbonate shells were pitted with holes.
Carbon dioxide reacts with seawater to make carbonic acid. Deep seawater naturally has more carbon dioxide, and less oxygen, simply because it is old. It has been breathed for centuries by everything alive in the ocean without remixing with oxygen from the surface. These frigid depths are a natural source of acidic water that wells up from time to time. When the water is acidic enough it dissolves calcium carbonate of the sort that makes up pteropod shells.
‘In the Southern Ocean, that usually happens at 750 meters down,’ Bednaršek told me. The depth is important, as Antarctic pteropods typically dive no deeper than 400 meters. ‘But we found the dissolution was starting to happen at 125 to 375 meters.’
the process works the same whether the carbon dioxide is from volcanoes or Volvos
She attributes this rising corrosive horizon to the additional input of anthropogenic carbon dioxide. When more carbon dioxide goes into the atmosphere than is removed, the balance not only traps infrared radiation that warms the air, but also makes the ocean more acidic (as the paleontologist Peter Ward likes to say, the process works the same whether the carbon dioxide is from volcanoes or Volvos).
According to some models, by 2050 this rising brew of more acidic water will reach the surface waters of the Antarctic, and calcium carbonate will begin to dissolve throughout much of the Southern Ocean.
‘It’s not a question of if pteropods will be dissolving, or if they will be compromised – it is certain they will be,’ Bednaršek said.
Her colleague Richard Feely at the National Oceanic and Atmospheric Administration’s (NOAA) Pacific Marine Environmental Laboratory in Seattle says that the dissolution of pteropods and other calcifying plankton could jam the biological carbon pump, a crucial part of the Earth’s living thermoregulatory system.
‘We would expect that it will get more and more difficult for the ocean to take up carbon dioxide the more carbon dioxide we add,’ Feely told me. ‘At this point, it’s still something of an open question, but it’s the foremost question marine scientists are working on now.’
Feely’s own interest is more than academic. From his office in America’s Pacific Northwest, he has watched with mounting alarm as more acidic waters have welled up from the deep ocean. It is the same corrosive water mass that recently wiped out oyster farms in Puget Sound.
The flip side of a more acidic ocean is a warmer one, and this too has consequences that could shake up the food web, beginning at the bottom – and again, the first omens of this phenomenon can be seen in the Antarctic. Though Antarctic sea ice has in fact grown in recent years (perhaps in response to changing weather patterns), the loss of ice cover in previous years has had a devastating effect on krill, the bedrock of the Antarctic ecosystem. Krill feed on the green algae that coats the ceiling of sea ice that floats atop the Southern Ocean. As ice declined in recent decades, krill saw their populations drop by as much as 80 per cent. They’re being replaced by weedier, barrel-shaped jelly animals called salps that thrive in less productive waters. But unlike krill, salps don’t sate the hunger of seals, whales, and penguins.
The effects of warmer water have begun to show up away from the poles. At NOAA’s Narragansett Laboratory in Rhode Island, Feely’s colleague Kevin Friedland has watched wild swings in ocean temperatures wreak havoc on the bottom of the food chain. One of the most ecologically and economically important species of zooplankton in New England is a cold-water shrimp-like crustacean called calanus finmarchicus. It is one of the copepods – a group that rivals krill for comprising the most biomass on planet Earth – and it is the lifeblood of the centuries-old New England fishing industry. It is also a tremendous conveyor of carbon to the deep ocean.
New England saw the warmest sea surface temperatures in its recorded history in 2012 and, as a result, 2013 was a catastrophic year for plankton. At his office in Rhode Island, Friedland showed me a graphic on his computer of the chlorophyll levels in the Northwest Atlantic as captured from space, where different intensities of green marked the success or failure of phytoplankton over the years. Last year’s bloom was a dull brown.
‘There was basically no bloom at all,’ he said.
After the phytoplankton failed to arrive in the spring, so did the grazing copepods, which appeared in their smallest numbers ever. While the extremely warm water of 2012 was likely an aberration that only briefly upset the physical conditions that typically prevail off the coast of New England, a long-term trend is nevertheless unmistakable: the bloom is coming earlier and earlier every year.
‘Fish stocks time their reproduction to the bloom,’ Friedland told me. ‘If the bloom starts moving around – if things are out of synch – that could be problematic.’ It likely already is. Despite drastic cuts to cod quotas in recent years, the fish have failed to rebound in the Gulf of Maine after decades of overfishing.
And the copepods are on the move. Just as warmer water fish have taken up residence in more northern climes – for example, hake in Maine or mackerel in Iceland – in the North Seacalanus finmarchicus is giving ground to a less desirable cousin from warmer water, calanus helgolandicus, whose life cycle is not co‑ordinated with that of local fish, causing chaos for fisheries managers. Zooplankton in the area has shifted its range by 10 degrees latitude northward in recent decades, a migration that moved it some 700 miles closer to the pole.
Warm-water zooplankton is typically smaller than the colder-water zooplankton it replaces. This is because warm water holds less oxygen and is more stratified, meaning nutrients are less likely to cycle to the surface and animals have to do more with less. Being smaller, these animals produce smaller packages of carbon to deliver to the sea floor. It’s possible that this process could limit the efficiency of Earth’s biological carbon pump, setting up a nasty feedback loop in the process.
the loss in productivity in the surface waters will reduce the animals on the ocean’s bottom by a mass equivalent to the entire human population
For the ghoulish creatures that inhabit the frigid depths of the ocean floor, far removed from the tumult taking place at the surface, this reduction of carbon flux could prove devastating. Despite a life spent in perpetual midnight – the sun a half-remembered myth from evolutionary history – these animals still run on the solar power generated miles above, which eventually snows down as consumable organic matter. One study estimates that by century’s end, the loss in productivity in the surface waters will have reduced the animals on the ocean’s bottom by a mass equivalent to the entire human population.
This shift in the carbon cycle is unprecedented in recent history, but something like it might have happened before in the planet’s deep history. James Zachos, an palaeoceanographer at the University of California Santa Cruz, found one such echo in the fossil record by travelling to the Walvis Ridge off the coast of Namibia, where he drilled deep into the Earth and pulled up a series of sediment cores – long chalky tubes made up of thousands of years’ accumulation of marine snow. They all bore the striking signature of an ancient acidifying ocean: an abrupt dark layer of dusky red clay where calcium carbonate had been dissolved away by more acidic water.
Though it happened during a spell that lasted more than 1,000 years – compared with the decades-long time-scale of the modern eruption of carbon dioxide – a curiously gigantic discharge of CO₂ appears in the fossil record coincident with these unusual clay layers 55 million years ago. It is suspected that the carbon came from underwater volcanoes in the North Atlantic that melted frozen methane deposits (methane, itself a greenhouse gas, degrades to carbon dioxide in the atmosphere). This event, the Palaeocene-Eocene Thermal Maximum (PETM), sent temperatures soaring and alligators scurrying, up to the balmy northern shores of Canada. In the oceans, up to a half of all single-celled calcium carbonate-secreting animals (called foraminifera) at the ocean’s bottom went extinct. Yet for all this ecological reorganisation, the PETM did not result in any other mass extinctions, which would be comforting, except that some attribute it to the longer timescale involved.
‘The difference between then and now is the rate,’ Zachos told me. And the rate, it turns out, is everything. How quickly carbon dioxide levels spike is much more important than the total volume of carbon released in determining the severity of an acidification episode. ‘In some ways the PETM is sort of the best analogy to today in terms of the rate of carbon emissions, the extent of climate change and the changes in ocean carbonate chemistry’ he said. ‘Having said that, the rates of change back then were about an order of magnitude slower than anthropogenic changes.’
The planet was also rescued, strangely enough, by the beneficent, haunting effect of long-dead plankton. It turns out that even in death, plankton can support life, by providing an emergency brake for a planet careening dangerously out of control. Like today, the surface waters of the PETM absorbed gigatons of carbon dioxide. When the oceans turned over, these more acidic waters eventually reached the bottom, and the corrosive water dissolved the thick seams of dead plankton lying in repose on the sea floor. The result was something like an antacid settling a dyspeptic stomach. The dissolution of the carbonate shells acted as a buffer, balancing the ocean’s pH so that within 100,000 years, the oceans were once again saturated with calcium carbonate.
‘The turnover time of the ocean is about 1,000 years,’ Zachos told me. ‘But most of the anthropogenic CO₂ is accumulating in the upper ocean within 100 years. The ocean can’t mix it fast enough into the deep sea.’ By 2050, Zachos expects the ocean’s pH to drop by the same amount as during the entire PETM. Despite these whiplash changes, Zachos expects that the ocean’s chemistry will be restored eventually, even if it takes more than 100,000 years. But no one knows what will happen in the short term. Perhaps evolution or animal physiology will surprise us, keeping up with the dramatic changes afoot. Or maybe there will be a mass extinction. All anyone knows is that everything is in flux.
At the end of our chat, Peter Wiebe gave me a handful of studies on zooplankton to read and wished me well, eager to get back to his time-worn journals documenting his life on the sea. I brace myself for the cold and stepped back out into the storm. The streets were blanketed in white and the downtown was silent. For the time being, the snow was still falling – above and below the waves.