Picture someone washing their hands. The water running down the drain is a deep red. How you interpret this scene depends on its setting, and your history. If the person is in a gas station bathroom, and you just saw the latest true-crime series, these are the ablutions of a serial killer. If the person is at a kitchen sink, then perhaps they cut themselves while preparing a meal. If the person is in an art studio, you might find resonance with the struggle to get paint off your hands. If you are naive to crime story tropes, cooking or painting, you would have a different interpretation. If you are present, watching someone wash deep red off their hands into a sink, your response depends on even more variables.
How we act in the world is also specific to our species; we all live in an ‘umwelt’, or self-centred world, in the words of the philosopher-biologist Jakob von Uexküll (1864-1944). It’s not as simple as just taking in all the sensory information and then making a decision. First, our particular eyes, ears, nose, tongue and skin already filter what we can see, hear, smell, taste and feel. We don’t take in everything. We don’t see ultraviolet light like a bird, we don’t hear infrasound like elephants and baleen whales do. Second, the size and shape of our bodies determine what possible actions we can take. Parkour athletes – those who run, vault, climb and jump in complex urban environments – are remarkable in their skills and daring, but sustain injuries that a cat doing the exact same thing would not. Every animal comes with a unique bag of tricks to exploit their environment; these tricks are also limitations under different conditions. Third, the world, our environment, changes. Seasons change, what animals can eat therefore also changes. If it’s the rainy season, grass will be abundant. The amount of grass determines who is around to eat it and therefore who is around to eat the grass-eaters. Ultimately, the challenge for each of us animals is how to act in this unstable world that we do not fully apprehend with our senses and our body’s limited degrees of freedom.
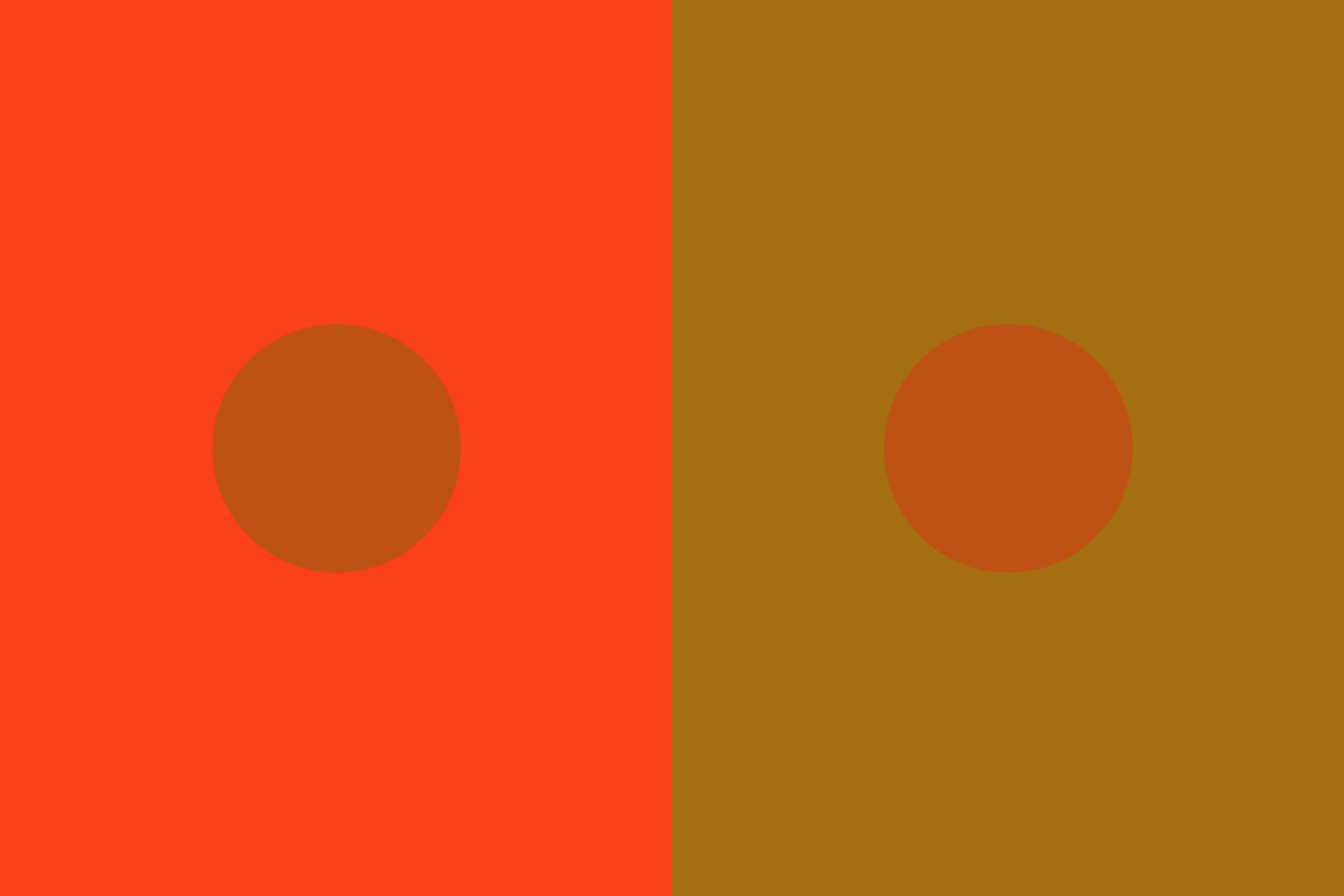
There is a fourth constraint, one that isn’t typically recognised. Most of the time, our intuition tells us that what we are seeing (or hearing or feeling) is an accurate representation of what is out there, and that anyone else would see (or hear or feel) it the same way. But we all know that’s not true and yet are continually surprised by it. It is even more fundamental than that: you know that seemingly basic sensory information that we are able to take in with our eyes and ears? It’s inaccurate. How we perceive elementary colours, ‘red’ for example, always depends on the amount of light, surrounding colours and other factors. In low lighting, the deep red washing down the sink might appear black. A yellow sink will make it look more orange; a blue sink may make it look violet. If, instead of through human eyeballs, we measured the wavelengths of light coming off the scene with a device called a spectrophotometer, then the wavelength of the light reflected off that ‘blood’ would be the same, no matter the surrounding colours. But our eyes don’t see the world as it really is because our eyes don’t measure wavelengths like a spectrophotometer.

Ulyanovsk, Russia, 1990. Photo by Peter Marlow/Magnum Photos
Dale Purves, the George B Geller Professor of Neurobiology (Emeritus) at Duke University in North Carolina, thinks that, because we can never really see the world accurately, the brain’s primary purpose is to help us make associations to guide our behaviour in a way that, literally, makes sense. Purves sees ‘making sense’ as an active process where the brain produces inferences, of which we are not consciously aware, based on past experiences, to interpret and construct a coherent picture of our surroundings. Our brains use learned patterns and expectations to compensate for our imperfect senses and finite experiences, to give us the best understanding of the world it can.
Purves is the scientist’s scientist. He pursues the questions he’s genuinely interested in and does so with original approaches and ideas. Over the years, he’s changed the subject of his research multiple times, all with the intent of understanding how the brain works, tackling subjects that were new and unfamiliar to him, as opposed to chasing trends and techniques or sticking to a tried-and-true research path. His career is an instance of the claim Viktor Frankl makes in Man’s Search for Meaning (1946): ‘For success, like happiness, cannot be pursued; it must ensue, and it only does so as the unintended side-effect of one’s personal dedication to a cause greater than oneself …’ If success is measured by accolades, then it did indeed follow Purves’s pursuits. Among a laundry list of awards and honours, he’s one of the few scientists elected both to the National Academy of Sciences (1989) and to what is now the National Academy of Medicine (1996). Election to either is considered to be among the highest honours that can be bestowed on a scientist in the United States. Nevertheless, if the name ‘Dale Purves’ sounds familiar to you, it is likely because you took a neuroscience course in college, for which the textbook was Neuroscience by Purves et al (one of the most popular and now in its 7th edition). Indeed, this is the text I used when I taught an introductory neuroscience course at Princeton.
Oddly enough, Purves’s passion for neuroscience took time and experience to materialise. As an undergraduate at Yale, he struggled initially but found a major – philosophy – intending to pursue medicine. Purves developed an interest in science but didn’t know anything about being a scientist, and medicine seemed close enough. In 1960, he entered Harvard Medical School thinking he would become a psychiatrist. In his first year, he took a course on the nervous system, taught by young hotshot neuroscientists, some of whom would go on to be among the greats of the 20th century (and whose work is now textbook material): David Potter, Ed Furshpan, David Hubel and Torsten Wiesel (the latter two won the Nobel Prize in 1981, along with Roger Sperry). Purves finished his medical degree in 1964, but he had grown disillusioned with psychiatry. He’d tried switching to general surgery, but realised he lacked the intensity of interest in surgery required to excel.
It was 1965, and the Vietnam War gave him time to think. Purves was drafted but, as a physician, could serve his time as a Peace Corps employee, which he did in Venezuela. There, he said, he came across a book, The Machinery of the Brain (1963) by Dean Wooldridge, that synthesised what he learned years ago in that first-year course on the nervous system. The book was written for the lay reader and shared the current knowledge about the brains of humans and other animals. Its particular angle was to compare the brain to the computer technology of the time. The book reignited Purves’s interest in the brain, which was first piqued in his medical school course. When he returned to the US, Purves would begin again as a researcher in neuroscience.
There were more neurons in the developing embryo than there would be in the adult chicken
I was an undergraduate at the University of Idaho when I first read Purves’s work. It was the early 1990s and I was a philosophy major like Purves but was really interested in neuroscience. So I sought hands-on research experience in a professor’s lab in the biology department. We were studying the immune-system markers of motor neurons in the rat’s spinal cord, the ones that connect to leg muscles causing them to contract. Mark DeSantis, my advisor, suggested a book by Purves. At the time, there were few courses or serious books on the brain. Purves’s Body and Brain: A Trophic Theory of Neural Connections (1988) was perfect, just what I needed. Its central thesis is that the survival of neurons making connections, and the number of these connections, is regulated by the targets of those neuronal connections. In essence, he was telling us that, unlike the static circuit board of a computer, which is carefully designed and built according to a plan, the circuits of the nervous system are constructed on the fly in accordance with signals it receives from the targets of its connections. Those targets could be other neurons, organs or muscles in the body. As a result, as the body changes over the course of development in an individual or in the evolution of species, the neural circuits will adjust accordingly. Why is this important? Purves showed us that the brain is more than just a controller of the body: it is also an organ system that is embedded in a dynamic relationship with the rest of the body, affected by body size, shape and activity.
Courtesy the Human Connectome Project
One of Purves’s favourite examples to illustrate what sparked this theory is from the work of one of his scientific heroes, Viktor Hamburger. Hamburger had been studying central nervous system development in the 1930s, first at the University of Chicago, then as a faculty member at Washington University in St Louis. Using chicken embryos and those spinal cord motor neurons, Hamburger showed that there were more neurons in the developing embryo than there would be in the adult chicken. How could that be? Why were there more neurons in an embryo than actually needed and why did some die off? Hamburger’s idea was that the muscles targeted by those neurons supplied a limited amount of trophic, or nutrient, factors. In essence, the target muscles were producing a ‘food’ (we now call it ‘nerve growth factor’) that kept the neuron alive. The size of the target determined how much food was available and therefore how many neuron lives it could sustain. Exploiting the ease with which chick embryos could be manipulated, Hamburger showed this by first amputating one of the wing buds (ie, the nascent wing). When he did so, the final number of motor neurons on the amputated side was lower than typical, with fewer on the ‘control’ side of the spinal cord. So, the limb bud was important for the survival of neurons. If that was true, then more of this ‘target tissue’ should save more neurons. Was it possible to ‘rescue’ those extra neurons that would normally die off? To answer this question, Hamburger surgically attached an extra limb bud on one side of the embryo, thereby artificially creating more target tissue. The result: more motor neurons survived on that side of the spinal cord. In both experiments, the size of the connection target – the body, those limb buds specifically – determined the number of neurons that survived. Purves ran with this idea of a dialogic relationship between body and brain.
Around four decades later, in the 1970s and ’80s, and Purves was now a young faculty colleague of Hamburger, his elder statesman at Washington University. Here, he took Hamburger’s theory about neuron-to-muscle connections and applied it to neuron-to-neuron connections. While he also looked at neuronal cell survival like Hamburger did, Purves also investigated the elimination and elaboration of individual connections that neurons make, their synapses. This was a big leap because Purves was now testing whether or not Hamburger’s findings about death and survival of neurons in the chick embryo were peculiar to that species’ neuron-to-muscle relationship. Was the same process apparent in other developing circuits in other animals? And, if so, if a neuron survives, then are the number of connections (those synapses) also subject to competition for trophic factors?
Neurons come in all shapes and sizes, with different degrees of complexity. If you’ve seen a typical picture of a single neuron then you know it looks like a tree or bush, with a set of root-like branches on one end and a single, long limb-like branch on the other. The latter can branch as well, depending on circumstances. One of those sets of branches – the dendrites – receives inputs from other neurons, and the other – the axon – sends outputs to other neurons. Together with his graduate student Jeff Lichtman, Purves wanted to know how synapse numbers change with development and across different species of animals. Purves and Lichtman started with a simple neuron-to-neuron connection, where the receiving neuron had zero dendrites and the sending neuron’s axons made synapses directly on the receiving neuron’s cell body. To see this, they would surgically remove a tight group of functionally similar neurons, known as a ‘ganglion’, from different animals. They would then carefully fill a few individual neurons with a special enzyme. When this enzyme is then given a chemical to react with, it produces a colour. This colouring allows the neuron to be visualised under a microscope in its full glory – all their branches can be seen and counted. (Imagine a microscopic glass-blown tentacled creature being filled with ink and then counting its appendages.)
The brain and body constitute a single, coherent and dynamic network; there is no way to separate them
The end of each branch represents a synaptic connection. Comparing connections in developing rats versus adults, they found that neurons initially received a few synapses from a number of different neurons. In a sense, the circuits were tangled up in young rats. By the time the rats were adults, each neuron had many synapses but only from one neuron – the circuit was untangled. How did this happen? Akin to the process of eliminating extra neurons based on target size, there was a process of elimination of superfluous connections from some neurons. Then there was an additional process of multiplication (or elaboration) of connections coming from the ‘correct’ (so to speak) neuron. In essence, once neurons could find the right partners by getting rid of the less desirable ones, their relationship could blossom in the form of additional synapses. Purves and Lichtman then replicated this basic finding with increasingly complex sets of neurons and in other species.
Before we get lost in the weeds, here’s the bottom line: trophic interactions between neurons match the number of neurons to the target size, and these interactions also regulate how many synapses they make. The grander theory is this: each class of cells in a neural pathway is supporting and regulating the connections it receives by trophic interactions with the cells it’s connected to down the line. Thus, a coordinated chain of connectivity extends from neuronal connections with the body’s muscles and organs to connections among neurons within the brain itself. The brain and body constitute a single, coherent and dynamic network; there is no way to separate them. They depend on each other at every level.
Some artists go through distinct periods in their careers, while others stick to similar themes in and/or approaches to their work for decades. Scientists are the same. Most stick to trying to answer one particular question, getting deeper and deeper, as they figure out more and more details about their subject. Others find that, at some point, they are satisfied with the answers at hand and move on, finding a new question or challenge. Purves is the latter type of scientist, making multiple radical shifts in his scientific research. His important work supporting trophic theory had an obvious direction for continued investigation: using molecular tools to find new ways to visualise synaptic development. Purves was not interested. His research programme up to this time exploited easily manipulated and unambiguously visualised neural circuits in the peripheral nervous system. The brain itself is a different story, there where all the important action supposedly is; its complexity and density make it impossible to address the same questions – which connections are disappearing or multiplying – with the same degree of clarity and specificity.
Neuroscience was changing as well. By the time the late 1980s and ’90s rolled around, the most attention-getting work focused on the brain, particularly in the neocortex – the part that has disproportionately increased in size in primates like us. Many who were interested in how the brain developed were inspired by those Nobel Prize-winners, Hubel and Wiesel, who elegantly demonstrated that the visual part of the neocortex had a critical period of development. At this point, Purves had reached middle age, and he was at an impasse. The answer to the question ‘What to do next?’ was not self-evident. As an academic scientist, one can pretty much study whatever one wants, but it has to be interesting, potentially consequential, and, for Purves especially, it has to be tractable: you should be able to formulate a clear hypothesis that could lead to an unambiguous finding. The answer came in the form of a new collaborator, Anthony-Samuel LaMantia, who joined his lab in 1988 as a postdoctoral fellow after completing his PhD on the development of the neocortex. Together, Purves and LaMantia decided to tackle the question ‘How does the brain grow?’
Mice were not born with their full set of glomeruli; over the course of development, new ones were added
There are many different kinds of brains, as many as there are animals. There is a beauty in all of them, perhaps because they adhere quite nicely to the form-follows-function principle of design. The designer in each case is natural selection’s influence on how a species develops, and thus the form its body and brain take in response to environmental challenges. Brain scientists study the anatomy of these solutions when we use any number of techniques like tracers, stains, imaging, etc. Each technique is suited to looking at the brain at a particular spatial scale. Consistently, what they reveal is that the brain is beautiful, sometimes stunning. At one of those scales, you can see repeated patterns of neural circuitry, or modules, that look exactly like the spots and stripes we see on the skin of so many animals. For example, depending on what dyes you use to stain it, the visual cortex of primates has a pattern of stripes, with each stripe seemingly dedicated to the visual signals coming from one of our two eyes. Stain it another way, and you’ll see an array of ‘blobs’ that are claimed by some to be dedicated to colour processing. Other animals have different patterns: rats have an array of barrel-shaped modules in their somatosensory (touch) cortex which corresponds to their array of facial whiskers. Dolphins have blobs in their auditory cortex; we don’t know what their function is.
Purves wanted to know how these iterated patterns of neural circuitry developed. He started by first looking just outside the neocortex, in the olfactory bulb of the mouse. In this structure, mice have a number of modules known as ‘glomeruli’. The olfactory bulbs of mice jut out from the main part of the brain and so are more accessible for experiments. Purves and LaMantia developed a method for exposing the bulbs in a live animal and staining the glomeruli with a dye that would not hurt the mice. They could then see that mice were not born with their full set of glomeruli; over the course of development, new ones were added. This was exciting and surprising because many popular theories at the time argued that brain development is mainly the result of selecting useful circuits from a larger repertoire of possible circuits. Here, they were showing that useful circuits were actually being constructed, not selected. Moreover, if circuits were constructed in this way after the animal is born, then the circuits might be influenced by experience. Were other modules in other species and brain areas added in the same way? In the macaque monkey visual cortex (ie, the experimental animal most closely related to humans and the brain area that is among the most studied) they couldn’t look at module development like they did in the mouse (looking at the same brain structure in the same animal repeatedly over time), but they were able to count the number of blobs in young monkeys versus adult monkeys. Unlike the glomeruli in mice, however, the number of blobs remained constant over time.
To Purves, this was not super exciting. He had hoped to find more traction on perhaps a new process of neocortex development in primates, one that he could elaborate into a novel research programme. Nevertheless, he did come to one important conclusion. It seemed that most scientists – indeed, many luminaries of neuroscience – wanted to see brain modules as fundamental features of the neocortex, each serving a particular behavioural or perceptual purpose. For example, one ‘barrel’ in the rat’s touch cortex is there to process the inputs of one whisker on its face. Purves pointed out that iterated patterns of modules may be found in one species’ brain but absent in a closely related species. Moreover, he also noted that they don’t seem to be obligatorily linked to function. ‘Blobs’ are there in the human and the monkey visual cortex and are linked to colour-vision processing, but nocturnal primates with poor colour vision still have blobs in their visual cortex. So, the blobs do not seem to enable colour vision. Similarly, chinchillas have a barrel cortex like rats but don’t have the whisker movements of rats. Cats and dogs have whiskers but no related modules in their touch cortex.
Thus, it seems that, while the iterated patterns of the brain are beautiful, they are unlike modern architecture in that their beauty is not linked to function. So why then do they form at all? Here, Purves suggested that iterated patterns are the result of synaptic connections finding and relying on each other and then making more of those connections in pathways that are most active. In other words, the iterated patterns of the brain are epiphenomenal, the byproducts of the rules of neural connections and competing patterns of neural activity. Those activity patterns are generated by sensory inputs coming from the sensory organs – the eyes, ears, nose and skin. So seeing beautiful-looking patterns in the brain does not necessarily mean they were constructed for a particular purpose.
I first met Purves in 1993 when I was interviewing for graduate school after he had moved to Duke University. I had already read a lot of his work and was in awe of his contrarian instincts and pursuit of work that is out of the mainstream yet important. When I entered his office for my interview, I was extremely nervous but managed to ask about the portraits on his office walls. They were scientists. John Newport Langley, a 19th-century British physiologist who made important discoveries about neurotransmitters. He inspired the problems Purves tackled as a new professor. The aforementioned Viktor Hamburger was also there. He was a major figure in 20th-century embryology and also a good friend of Purves, despite the difference in their ages and experience. Another photo was of Stephen Kuffler, perhaps the most beloved figure in neuroscience at the time and who made key discoveries in vision. Kuffler had organised the neuroscience team who taught Purves when he was in medical school, and Purves considers him a mentor who exemplified what to pursue (and what not to pursue) in neuroscience. The final photo was of Bernard Katz, a Nobel laureate who figured out how neurons communicate with muscles. Purves collaborated with Katz in the 1970s and considers him a paragon of scientific excellence. I was admitted to Duke and, a year later, moved to Durham, North Carolina hoping to study with Purves or LaMantia, who was there too as a new professor.
When I arrived at Duke, Purves was about to make a major change, away from studying the brain itself entirely. This seemed kind of crazy after so much success with discoveries about the developing nervous system, building an enviable career and becoming a sought-after leader in the field. But Purves’s restless instinct arose again and he switched his focus, this time to study perception. He had a hunch that the great advances wowing people about brain anatomy and the function of circuits therein were not going to be enough to make it clear how the brain works in actually guiding human behaviours. The origin of the hunch was in philosophy, which Purves had majored in as an undergraduate. The philosopher George Berkeley (1685-1753) had noticed that our eyeballs take in radically different-sized, three-dimensional objects and then project them back onto the retina (the sensory wall in the back of the eye) in exactly the same size and only in two dimensions (known as the inverse optics problem). This is why framing a distant human’s whole body between your two fingers, seemingly able to crush them, is amusing. It uses forced perspective to imply an impossibility. The implication of the inverse problem is profound. It means that the information about the object (the source) coming into our brain is uncertain, incomplete, partial.

Portrait of George Berkeley (1730) by John Smibert. Courtesy Wikipedia
As a solution to the inverse problem, the scientist Hermann von Helmholtz (1821-94) proposed that perception relied on learning from experience. We learn about objects through trial and error, and make inferences about any ambiguous image. Thus, since we have no experiences with lilliputian human beings, we can infer that the tiny human in the forced-perspective example is actually far away. Purves took the seed of Helmholtz’s idea – that our perception depends on experience – and built an entire research programme around it. Since the mid-1990s, he and his collaborators have systematically analysed a variety of visual illusions in brightness, contrast, motion, and geometry. They have shown that our perceptions are experience-based constructions, not accurate reflections of their sources in the real world. The example of ‘red’ from the beginning of this essay is based on his colour work.
Our ability to perceive accurately is largely based on past experiences and learned associations
Purves and his collaborator Beau Lotto would generate two identically coloured ‘target’ squares on a computer screen but give them backgrounds of different colours. The backgrounds would make the two squares look like they were different colours (even though they were actually identical, as measured by a spectrophotometer). Then, participants were asked to adjust the hue, saturation and brightness (the same controls on your phone’s camera app) of the target squares until they looked identical. Each participant’s adjustments were quantified and used as a difference measure between perception and reality. Ultimately, Purves’s research led to the conclusion that the brain functions on a wholly empirical basis. We construct our perception of the world through our past experiences in that world.
This is a radical departure from the long-standing prevailing orthodoxy that the brain extracts features from objects and other sensory sources only to recombine them to guide our behaviour. Instead of extracting features and combining them in the brain (red+round = apple), Purves argues that it is our learned associations among an event or feature of the world, the context in which it appeared and the consequences of our subsequent actions that builds our umwelt, our self-centred world. The research of Purves and his collaborators showed that our ability to perceive accurately is largely based on past experiences and learned associations. This means that we must learn about the space around us, the objects in it and other aspects of perception; these are not innate but developed through interaction with the environment. This all seems very reasonable. The environment is always in flux, with different challenges at different times for any animal. You wouldn’t want your brain fine-tuned to an environment in which you no longer live. Equally, it also wouldn’t make sense for a species to build each individual’s brain as a tabula rasa, to start from scratch with every generation.
Purves’s findings and interpretation lead to a more philosophical puzzle. To what extent is the ‘environment’ that the brain is trying to make sense of actually ‘out there’ beyond our heads? Is there a real reality? Purves has shown that, even if there is a real reality, we don’t perceive much of it… or at least we don’t have a universal way of perceiving it. For example, not all humans see the same colours the same way. There are two reasons for this. One is that colour and its interpretation depend highly on environmental factors. The other is that perception also depends on experience. Experiences depend on your interaction with specific environments. Do you live in the sea, on land, in burrows, in a nest or in a climate-controlled house? Do you have vision, or are you blind? What do your physiology and anatomy allow you to perceive and interact with? What have you seen before? Your perception and interpretation of the world, and indeed those of other animals, depend on the answers to these types of questions.
In her memoir Pilgrim at Tinker Creek (1974), Annie Dillard writes about coming across a book on vision, which shows what happens when blind humans of all ages are suddenly given the ability to see. Does experience really determine how we see and act in the world? That book was Space and Sight (1960). In it, Marius von Senden describes how patients who were blind at birth because of cataracts saw the world when those cataracts were removed. Were they able to see the world as we see it, those of us with vision since birth? No. Most patients did not. In one example from the book, Dillard recounts:
Before the operation a doctor would give a blind patient a cube and a sphere; the patient would tongue it or feel it with his hands, and name it correctly. After the operation the doctor would show the same objects to the patient without letting him touch them; now he had no clue whatsoever what he was seeing.
There is even an example of a patient finally ‘seeing’ her mother but at a distance. Because of a lack of experience, she failed to understand the relationship between size and distance (forced perspective) that we learn from experience with sight. When asked how big her mother was, she set her two fingers a few inches apart. These types of experiments (which have been replicated in various ways) show just how important experience and learned associations are to making sense of the world.
Today, Purves has enough studies to show an operating principle of the nervous system or – more cautiously, he would say – ‘how it seems to work’. The function of the nervous system is to make, maintain and modify neural associations to guide adaptive behaviours – those that lead to survival and reproduction – in a world that sensory systems cannot accurately capture. It is not a stretch to link Purves’s work, all the way back, on trophic theory to the current ideas. A biological agent must assemble, without any blueprint or instructions, a nervous system that matches the shape and size of a changing body. This nervous system, paired with sensory organs that filter the world in peculiar ways, must somehow process the physical states of the world in order to guide behaviour. Similar principles – neural activity, changing synaptic connections – that guided development also guide our ongoing perceptions of an ever-changing world. We use our individual experiences to do this guiding. If we happen to perceive or interpret events like other human beings, it is because we have similar bodies and shared similar experiences at some point. To my experience, Purves is a paragon of scientific excellence.
Purves first identifies a big, interesting question, and then finds whatever means are necessary to find the answer
Recently, I asked Purves how he thought of the arc of his career, and it was very different from my perception of it. From my perspective, it seems that Purves’s question is always ‘How is a nervous system built?’ Addressing this question took him to increasingly large scales: from trophic theory and neural connections in the peripheral nervous system to neural construction of the brain (iterated patterns; growth of the neocortex) to the relationships between brain-area size and perceptual acuity to the construction of ‘reality’ via experience. I asked him what he thought about this narrative, and he responded:
That is one way of framing it, but I don’t really see a narrative arc. As you know, one’s work is often driven by venal/trivial considerations such as what research direction has a better chance of winning grant support or addressing the popular issues of the day. The theme you mention (how nervous systems are built) was not much in my thinking, although in retrospect that narrative seems to fit.
Neither one of us is wrong. In fact, we are interpreting the body of work according to our own experiences and how it best suits our needs, just as his research would suggest.
Purves’s remarkable research insights are the product of his distinctive approach to science. A popular approach in neuroscience is to identify one problem early in a career and to just keep plugging away, learning more and more details about it. Then, maybe acquire an exciting new technique – like fMRI in the 1990s or optogenetics in the 2000s – to investigate the same problem in a different way. Or adopt the new technique and then search for a new question that could be answered by that method. Another approach would be to just apply the method, collect some data and only then ask what story can be told with those data. None of these approaches is ‘wrong’, but Purves’s scientific approach is in stark contrast to this. He first identifies a big, interesting question, one that could possibly have a ‘yes’ or ‘no’ answer, and then finds whatever means are necessary to find the answer.
To put it another way, there is a lot of thought behind his work and approach, and there is a lot of thought about what any findings may mean in the big picture of brain science. Purves is always engaged. Very few scientists have original, influential work in multiple domains of their field. Dale Purves has achieved major advances in our understanding of brain development, from small circuits to big ones, and from bodily experience to a new way of thinking about how the brain works.