I began exploring the concept of cellular memory – the idea that memory can be stored outside the brain, in all the body’s cells – after reading an article on Reuters headlined ‘Tiny Brain No Obstacle to French Civil Servant’ in 2007. It seems that a 44-year-old French man had gone to hospital complaining of a mild weakness in his left leg. Doctors learned that the patient ‘had a shunt inserted into his head to drain away hydrocephalus – water on the brain – as an infant. The shunt was removed when he was 14.’ When they scanned his brain, they found a huge fluid-filled chamber occupying most of the space in his skull, leaving little more than a thin sheet of actual brain tissue. The patient, a married father of two children, worked as a civil servant apparently leading a normal life, despite having a cranium filled with spinal fluid and very little brain tissue.
To my surprise, I unearthed in the medical literature an astonishing number of documented cases of adults who, as children, had parts of their brain removed to heal their persistent epilepsy. Following hemispherectomy – where half the brain can be removed to control seizures – most children showed not only an improvement in their intellectual capacity and sociability but also their apparent retention of memory, personality and sense of humour. Similarly, adults who have had hemispherectomies enjoyed excellent long-term seizure control and increased postoperative employability.
If people who lack a large part of their brain can function normally, or even relatively normally, then there must exist, I thought, some kind of back-up system that can kick in when the primary system crashes. I devoted the next six years to studying the medical and scientific literature searching for evidence to support my hunch.
We’ve long assumed that one of the fundamental functions of the brain is its ability to store memories, thus allowing animals, including humans, to alter behaviour in light of past experience. If the seat of all memory was truly the brain, then to ensure long-term stability of stored information, the brain cells and their circuits would need to remain stable, like the books on your bookshelf. If someone started to tear pages out from these books, not only would the books be seriously damaged but you would have lost forever these books’ contents.
Yet, animals such as the planaria that exhibit a remarkable capacity to quickly regrow new body parts, including their brains, confront us with a fascinating question: how can fixed memories persist when bodies and even brains do not? Animals that hibernate and undergo massive pruning of their cerebral neurons during the cold months confront us with a similar problem. Because, when they recover their strength and health in the spring, many of their previously learned behaviours return. The same holds for metamorphosing animals such as the common frog, which develops after a larvae becomes a tadpole. Caterpillars, meanwhile, go through five stages of growth. Memories formed in these animals in their earliest embryonic states survive extensive remodelling of their bodies including their brains, but how?
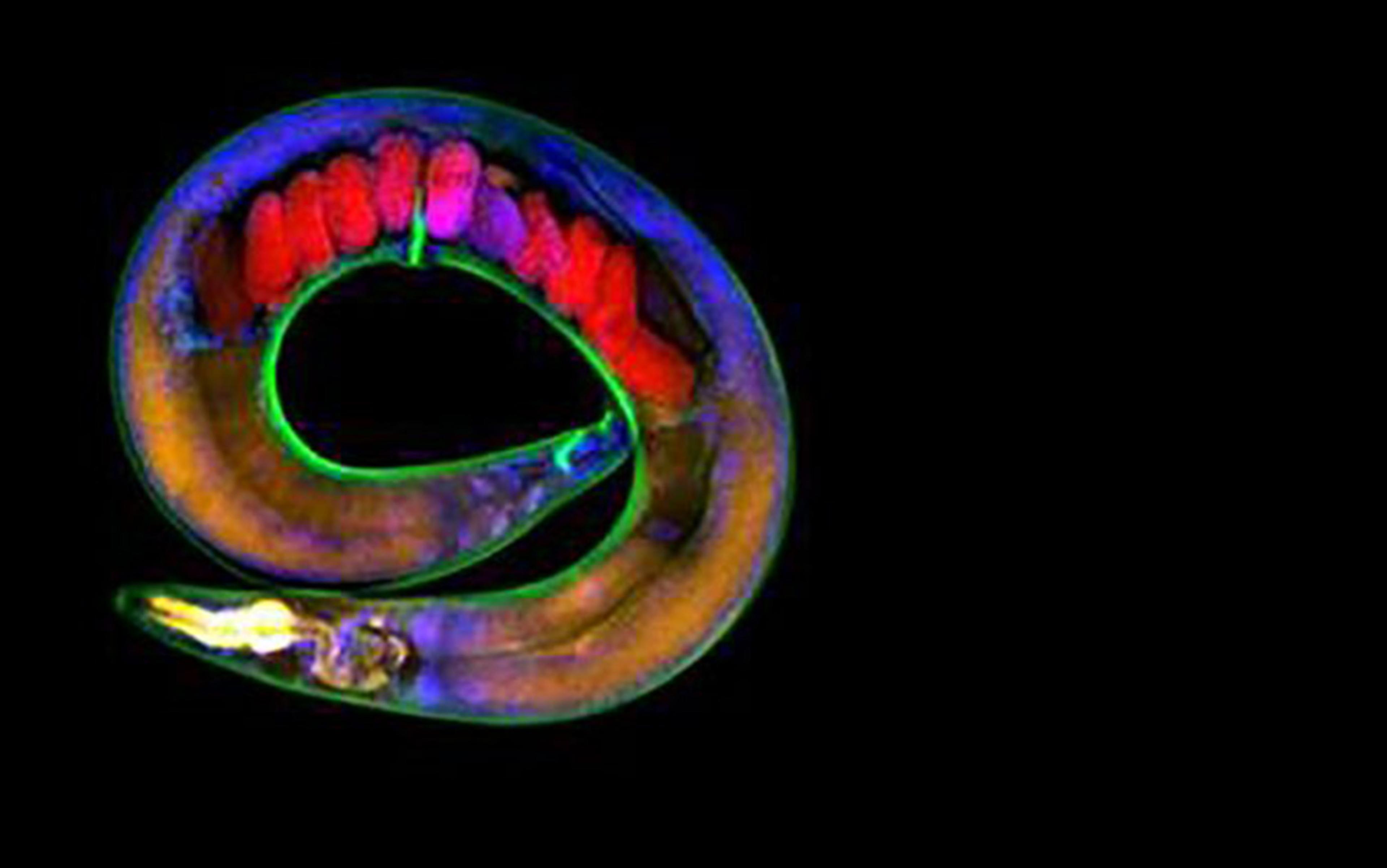
Because of their regenerative capabilities, planarian flatworms are one of nature’s gifts to science. Splitting a planarian down the middle quickly gives rise to two cross-eyed little worms staring back at you. Whole worms can regenerate from a small slice of the adult worm within a few days. Decapitation leads to the development of two new worms. Some biologists have succeeded in chopping up one planarian into 279 pieces. Each tiny piece eventually formed a miniature complete worm, which grew in time to its normal size of up to ¾ inches, depending on the species and the availability of food.
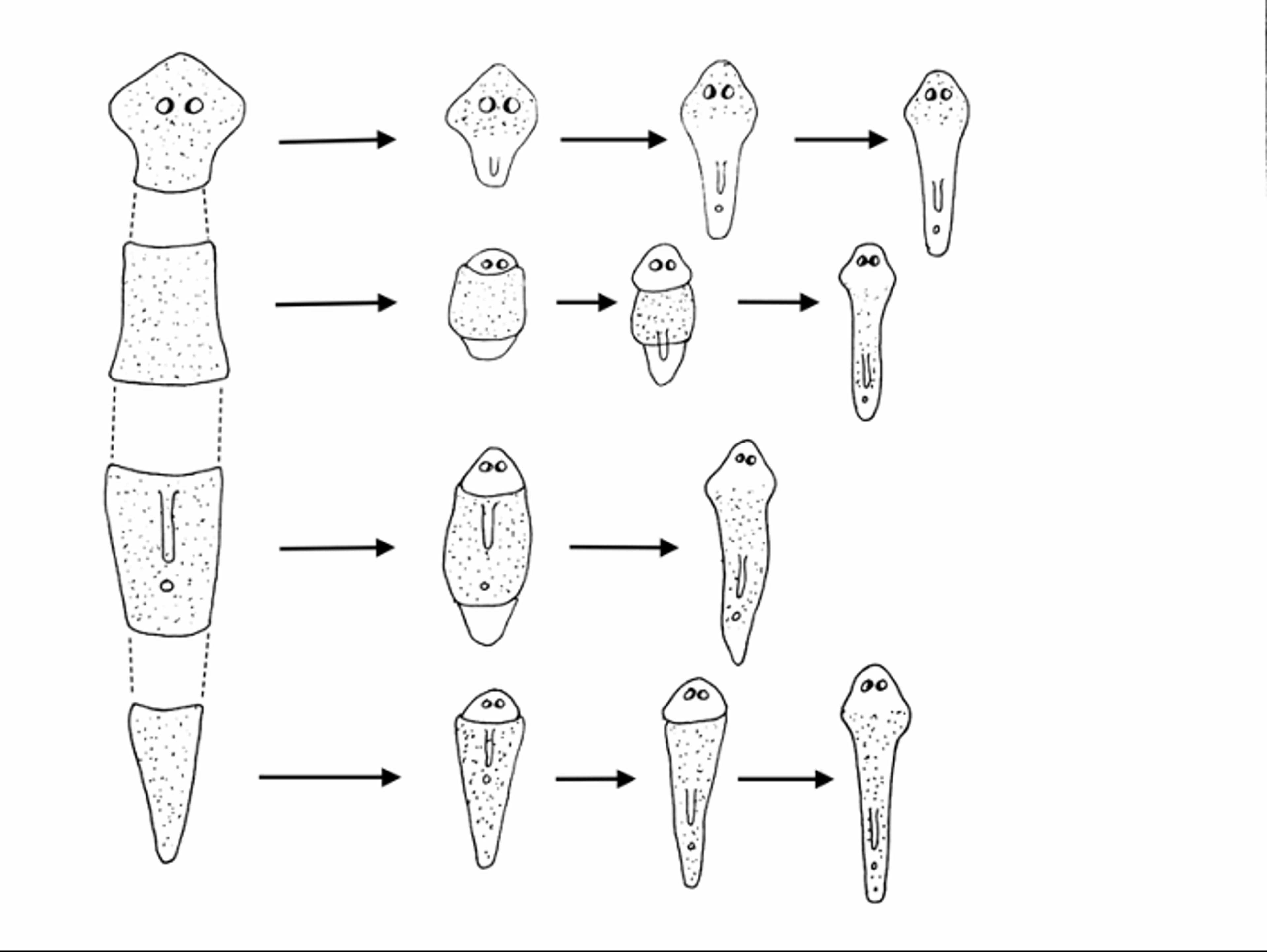
Figure 1. Planarian worm decapitation and regeneration sequence. Illustration by Bob Tadman
How do they manage such an incredible feat? A few years ago, researchers discovered that a resident population of adult stem cells (neoblasts) enable these worms to regenerate any body part after surgical removal of that part. However, as important as stem cells are, they cannot account for the persistence of memory after a planarian’s head is removed and its body grows a new head.
The planarian is one of the simplest animals living on this Earth, with a body plan of bilateral symmetry and head orientation. The brain of these flatworms has a bi-lobed structure with a cortex of nerve cells and a core of nerve fibres including some that connect the two hemispheres. Many structural features of planarian neurons including synapses are similar to human brains. Planaria are thus a popular organism for the study of memory.
This was evidence that memory in flatworms was not localised in the head but distributed throughout the body
In the 1950s and ’60s, the experimental psychologist James V McConnell and colleagues at the University of Michigan conducted studies using planarians to explore memory processes. In one series of experiments, planarians were trained to respond to certain stimuli, light and electric shock. When their heads were cut off and their bodies regenerated a new head, many of the regenerated worms demonstrated by their responses that they remembered their training.
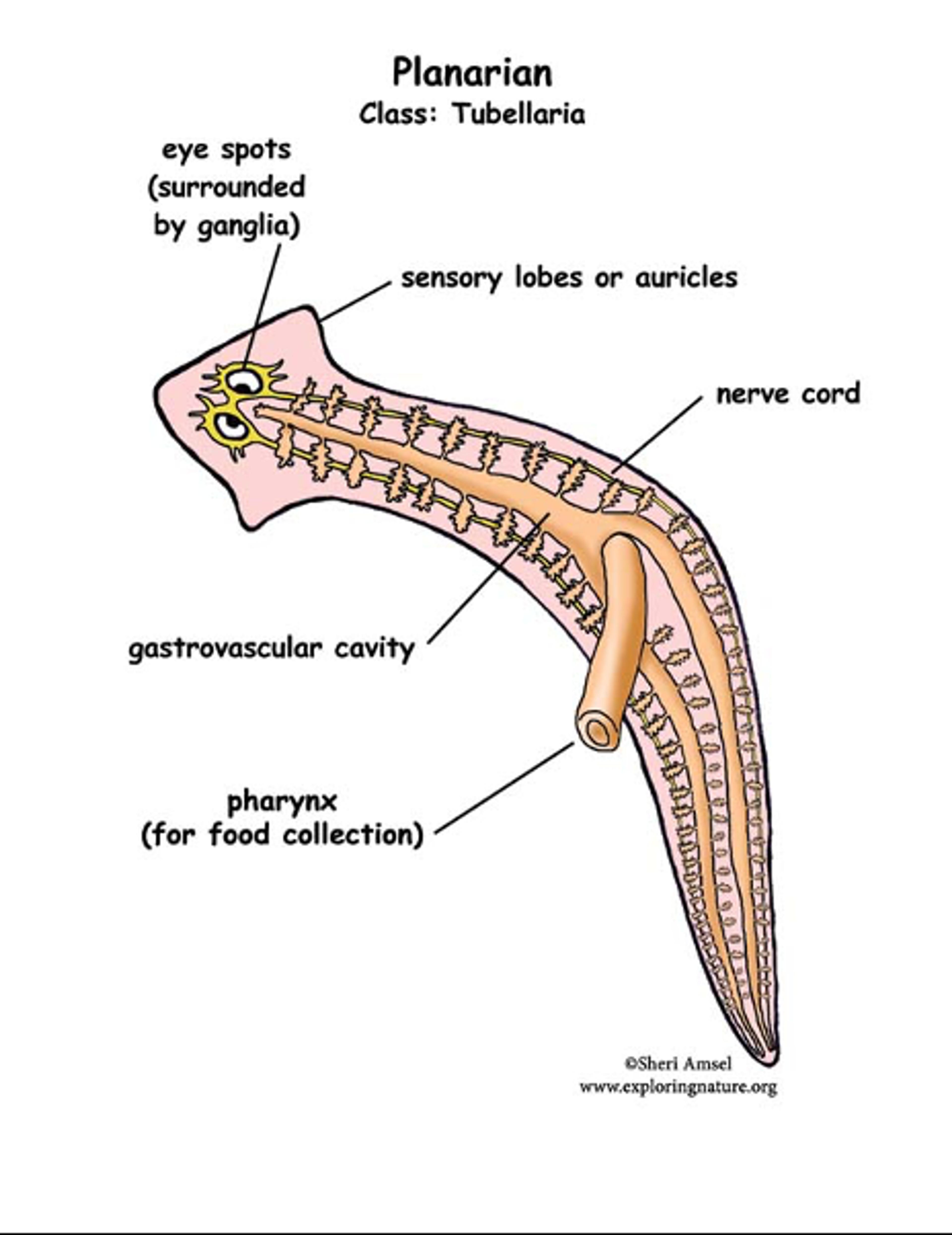
Figure 2. Planarian anatomy. Illustration © Sheri Amsel/Exploring Nature
In another series of experiments, planarians conditioned to associate a light signal with an unpleasant electric shock were ground up and fed to other planarians. These cannibal worms learned to respond to the light stimulus (by turning away from it) faster than a control group. McConnell interpreted this as evidence that memory in flatworms was not localised in the head but was distributed throughout the animal’s body.
A significant proportion of the scientific community did not trust these experiments, citing problems with the use of appropriate controls, observer bias and other reasons. But in 2013, a group led by the biologists Tal Shomrat and Michael Levin at Tufts University in Massachusetts published a paper that essentially supported McConnell’s findings and, by some reports, opened up a whole new can of worms.
Shomrat and Levin’s paradigm-shifting paper reported on their experiments on planaria of the species Dugesia japonica. The researchers took advantage of a quirk of planarian behaviour: once they get used to a familiar location, they will settle in to feed more quickly than planarians that find themselves in a new environment. Also, the worms naturally avoid light.
The researchers had one group of planarians living in containers with a rough-textured floor while the other group was housed in a smooth-floored Petri dish. After a few days, the worms were tested to see how readily they would eat liver in an illuminated quadrant on the bottom of a rough-textured dish. Automated video-tracking and subsequent computer analysis of the worms’ movements showed that the group that had spent time in the rough-floored containers overcame aversion to the light significantly more quickly and spent more time feeding in the illuminated space than did the non-familiarised group.
Both groups of worms were then decapitated. Their bodies were housed in a smooth-floored environment while their heads regenerated. Approximately two weeks later, the fully regenerated segments were again tested. Worms regenerated from the familiarised group were slightly but not significantly quicker to feed in the illuminated part of the container, demonstrating that they retained recognition of the link between this type of surface and a safe feeding environment.
However, the worms exhibited no learned behaviour prior to the regrowth of their brains. Evidently, the planarian needs to possess a brain for the behaviour to occur. The biologist Takeshi Inoue in Japan had hypothesised that the new brain is regenerated as a blank slate and is gradually imprinted by traces of the previous memory by the worms’ peripheral nervous system (which would have been modified during the training phase). The modified peripheral nervous system retrains the new brain in a short session, and that is sufficient to restore memory.
In their memorable 2013 paper, Shomrat and Levin suggested that traces of memory of the learned behaviour are retained outside the brain. But rather than assuming that this is accomplished by the peripheral nervous system, they believe that other mechanisms – those that are wired and rewired by experience – might be involved. They have indicated that all the major mechanisms by which nerves function, from neurotransmitters to electrical synapses, exist throughout the cells and tissues of the body, not just the brain.
Another period of great reorganisation for the brain is hibernation, a state of inactivity and metabolic depression in warm-blooded animals such as squirrels, hamsters, hedgehogs, polar bears and bats. Hibernation usually occurs in winter months, and is characterised by a general lowering of metabolic rate, body temperature, breathing and heart rate. Hibernation can last several days, weeks or months, depending on the species.
A good example of a hibernating mammal is the arctic ground squirrel. Every September in Alaska, Canada and Siberia, these squirrels retreat into burrows more than a metre beneath the tundra, curl up in nests built from grass, lichen and caribou hair, and begin to hibernate. Their core body temperature plummets, dipping below the freezing point of water. Hibernation devastates the central nervous system of these animals. Their neurons shrink, and thousands if not millions of vital connections between brain cells shrivel. Extensive pruning occurs of areas necessary for long-term memory such as the hippocampus. Upon recuperation, the majority of hibernating animals, including the European ground squirrel, demonstrate intact memory through kin recognition, identification of familiar as compared with non-familiar animals, and retention of trained tasks.
In an Austrian study, ground squirrels were trained in summer to successfully accomplish two tasks: a spatial memory task in a maze, and obtaining food from a feeding machine. In spring, the same tasks were repeated. The hibernating group did not do as well as the control group in performing the learned tasks. On the other hand, the animals were successfully able to discriminate familiar from unfamiliar individuals in their group. It is unclear why the animals could demonstrate memory in one realm but not others, though the authors speculate it could be the result of the complexity of the task or the brain region responsible for the memories. This study clearly demonstrates the persistence of social memories after considerable loss of neurons in the brain during hibernation.
Shrews decrease their braincases before winter by 15.3 per cent, then regrow by 9.3 per cent in spring
In addition to lowering their metabolic rate, another way that hibernating animals survive their long state of torpor is by selectively kickstarting a tissue-specific process called autophagy, which refers to cells literally destroying themselves, so the tissues the animal doesn’t need while hibernating are removed and thus no longer require maintenance.
When ground squirrels emerge in the spring after hibernating all winter, their guts have shrunk to about half their original weight, but their hearts remain unaffected because they need to keep beating, though at a much slower rate. But guts and neurons are not the only things that shrink. So do their testicles. When male ground squirrels wake up in the spring, their gonads have shrunk to almost nothing. But breathe a sigh of relief. They soon succeeded in regrowing them.
In a German study, Alpine marmots were trained to jump on two boxes or walk through a tube. When retested after six months of hibernation, their abilities were found to be unimpaired. The scientists conducting the study concluded that long-term memory in Alpine marmots is unaffected by hibernation.
Shrews are even smaller than marmots. While diminutive in size, when it comes to enduring the hardships of cold weather, they are biological giants. A new study from the Max Planck Institute for Ornithology in Germany used X-ray images to show that individual shrews decreased the size of their braincases in anticipation of winter by an average of 15.3 per cent. Braincases then partially regrew by 9.3 per cent in the spring. The dramatic changes in skull and brain size apparently did not adversely affect their post-hibernation behaviour.
Meanwhile, scientists affiliated with the Sensory Ecology Group at the Max Planck Institute decided to examine the memory retention of bats. They trained the bats to find food in one of three maze arms. After training, all bats performed 100 per cent correctly. Then they went into hibernation. When they ‘woke up’, the bats that had hibernated performed just as well as those that had not. The researchers concluded that bats benefit from an as-yet unknown neuroprotective mechanism to prevent memory loss in the hibernated brain. Biochemical studies on the brains of frozen wood frogs have revealed various neuroprotective factors implicated in the promotion of tissue survival. While all these factors probably play a role in preserving a small collection of neurons that will form the scaffolding for the growth of new neurons after the animal ‘wakes’, I don’t think they can possibly be responsible for preservation of complex memories.
No matter how you look at it, all these findings speak to the preservation of memory after hibernation.
Memories also survive metamorphosis, the process of transformation from the infant form to a juvenile and then an adult. As holometabolous insects traverse four life stages – from egg to larva to pupa to adult – they experience extensive neurogenesis, pruning and cell death in their brains. Despite these radical changes to their central nervous system, memories from earlier stages of their existence survive.
The adult moth’s brain contains about 1 million nerve cells. In comparison, the human brain has close to 100 billion. Yet there is a lot going on inside that pinhead moth brain. A team of researchers at Georgetown University in Washington, DC studied learning in a species of moth called the tobacco hornworm (Manduca sexta) (Figure 3, below).

Figure 3. The tobacco hornworm moth (Manduca sexta). Photo by Kugamazog/Wikimedia
The researchers exposed the larvae of this species to the smell of ethyl acetate (EA) paired with a mild electric shock. When offered the choice of fresh air or EA-scented air, adult caterpillars not preconditioned as larvae (Figure 4, below) showed neither attraction nor aversion to the odour of EA. Larvae exposed to shock alone showed no attraction or aversion to EA. But when caterpillars were preconditioned as larvae with odour prior to shock, 78 per cent of them chose fresh air instead of EA. Scientists say this is the first solid evidence we have of learning and memory surviving the later stages of metamorphosis in this species.

Figure 4. The tobacco hornworm pupa. Photo by Daniel Schwen/Wikimedia
Yukihisa Matsumoto at Hokkaido University in Japan was similarly able to demonstrate the existence of long-lasting aversive memory in the hemimetabolous cricket (Gryllus bimaculatus), which retained an association between an odour and salt water for up to 10 weeks. The learned preference was altered when they were given reversal training at six weeks after initial training. The researchers concluded that crickets are capable of retaining olfactory memory practically for their lifetime, and of easily rewriting it in accordance with experience. If only humans were that smart.
The question arises: if associative behaviour is indeed retained, does it truly result from the persistence of larval neurons through metamorphosis? Or is the reorganisation of the insect nervous system during metamorphosis so dramatic that it would preclude the persistence of chemosensory memory?
The memories of all our experiences, though not always accessible, persist etched into our embodied mind
Frogs that undergo metamorphosis offer a clue. The behavioural psychologist Peter G Hepper at Queen’s University Belfast in Northern Ireland injected frog eggs with one of two substances, orange or citral (an odour compound in citrus), respectively. After hatching, these tadpoles preferred to feed on food containing the substance they were exposed to when they were still eggs. Even more surprisingly, after the tadpoles metamorphosed into frogs, they maintained the acquired odour preference.
Meanwhile, working with wood frog larvae, researchers affiliated with the University of Saskatchewan and Missouri State University conditioned them to chemical cues from unfamiliar predators. When the frogs reached adulthood, they responded with fear to the same cues. Similarly, when eggs of ringed salamanders were exposed to chemical cues from predators, the larvae that hatched showed reduced activity and greater shelter-seeking behaviour. On the other hand, larvae that had been exposed to neutral cues did not show these behaviours. Since embryonic experience is a good predictor of the future conditions that the organism will encounter, it follows that learning associated with exposure to negative stimuli during development will be adaptive.
Finally, studies of other beetles, fruit flies, ants and parasitic wasps have repeatedly and convincingly demonstrated that larval experience instructs adult behaviour. As they metamorphose, animals experience major changes in body form, lifestyle, diet and the use of their senses. Is a maggot the same animal as the noisy blowfly, the caterpillar the same as the colourful butterfly? Are you the same person you were when you were born? The same person as when you were conceived? The same in some ways but also very different. Notably, despite the changes our bodies undergo as we age, the memories of all our experiences from conception on, though not always accessible, persist etched into our embodied mind.
Can stable memories really remain intact in animals, including humans, who undergo massive loss and rearrangement of their cerebral neurons? I align myself on this point with Levin, the biologist who in 2013 proposed planarians as a key emerging model species for investigating how memories are encoded in biological tissue and how they survive. The answers, when we find them, are likely to have important implications for treating degenerative brain disorders such as Alzheimer’s with stem cells. It seems credible to conclude that memory, in addition to being stored in the brain, must also be encoded in other cells and tissues in the body. In other words, we are all endowed with both somatic and cognitive memory systems that mutually support each other.
In aggregate, the evidence suggests that aspects of intelligence and consciousness traditionally attributed to the brain have another source as well. Our memories, our tastes, our life knowledge, might owe just as much to embodied cells and tissues using the same molecular mechanisms for memory as the brain itself. The mind, I conclude, is fluid and adaptable, embodied but not enskulled.
This essay is based on the book The Embodied Mind by Thomas R Verny, published on 5 October 2021 by Pegasus Press, New York.
To read more about memory and the mind, visit Psyche, a digital magazine from Aeon that illuminates the human condition through psychology, philosophy and the arts.