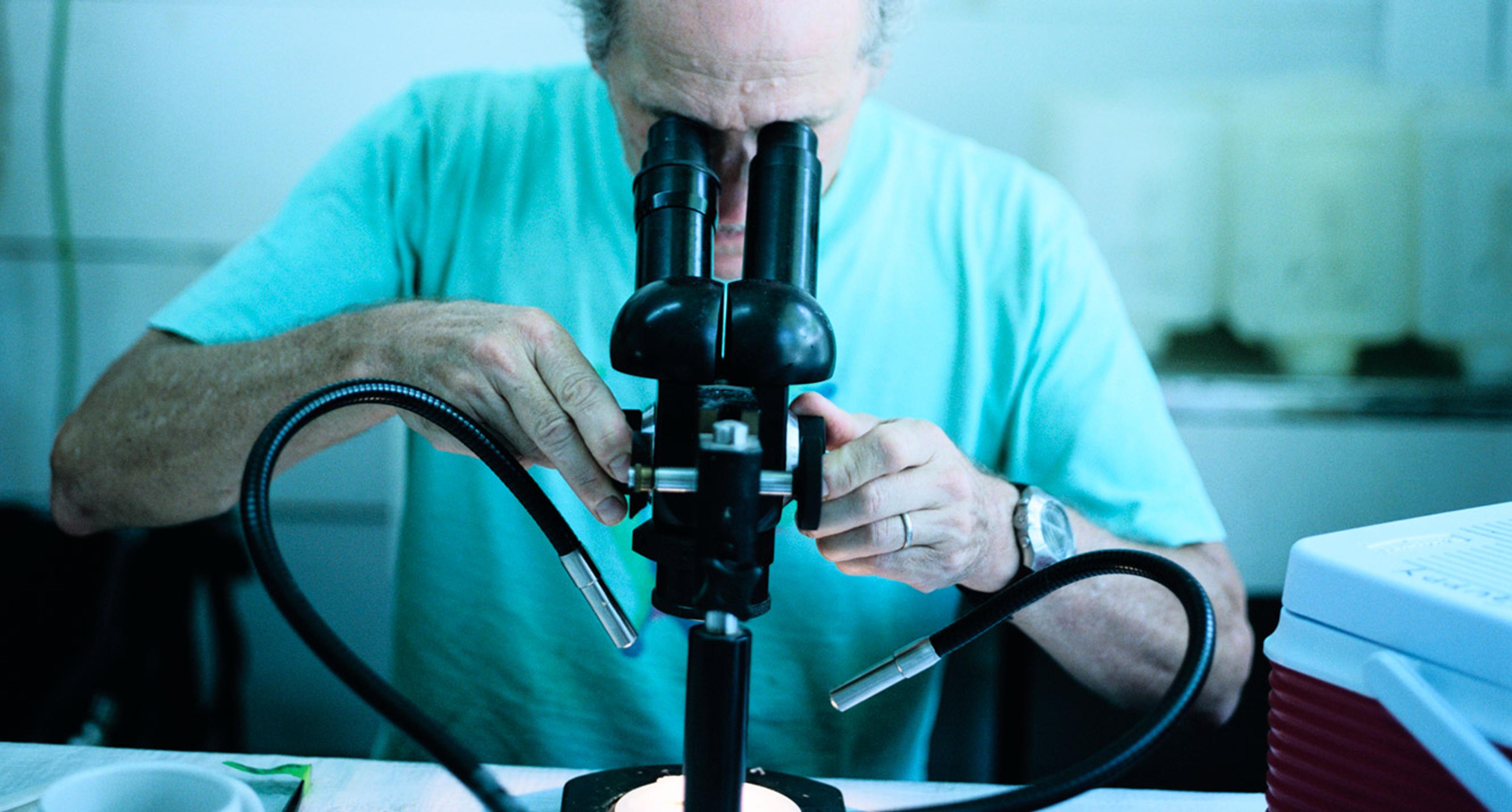
At the end of August 1939, the German archaeologist Otto Völzing discovered around 200 fragments of carved mammoth ivory at the back of a cave in southern Germany. With war just a week away, Völzing’s find was hurriedly collected in a box, where it lay unnoticed in a museum archive for decades. It wasn’t until the 1960s, when the shards were inventoried, that something astonishing emerged out of the heap of broken pieces. They formed an incomplete figurine, with a chimeric mix of features: the body of person, and the head and forearms of a cave lion. Subsequent excavations in the 1970s found further pieces of what has come to be called the Lion-Man of Hohlenstein-Stadel. Carved from a mammoth tusk around 40,000 years ago, it is one of the earliest examples of the human capacity to imagine forms that don’t exist in nature.

Lion-Man (Löwenmensch) from three angles. Carved from mammoth tusk. Discovered in the Stadel Cave, Baden-Württemberg, Germany. Dated to 40,000 years old. Copyright Ulmer Museum/photo by Yvonne Mühleis
Every age of human history since the Lion-Man has tried to think beyond nature. In Hesiod’s Theogony, composed around 700 BCE, the chimera was a compound being with the head of a lion, the body of a dragon, and a snake’s-head tail (and an extra goat’s head protruding from its back for good measure). In W B Yeats’s poem ‘The Second Coming’ (1920), a similar ‘rough beast’ is a harbinger of ruin.

A decorated plate featuring a chimera. Apulia, Italy, 350-340 BCE. Courtesy Louvre, Paris
In our own time, the chimeras are something different. Living things in every part of the biosphere have been forced by climate change, pollution and the spread of non-native species to adapt their bodies and behaviours to a human planet. They may not have visibly merged forms like the Lion-Man, but they do bear the impression of another species: us.
It wasn’t our intention that humanity would become the planet’s greatest evolutionary force; yet the fact that we are confronts us with an urgent and difficult question. Some animals, plants and insects can adapt but, for many, the pace of change is too great. Should we try to save them by deliberately intervening in their evolution?
The emergence of precision gene-editing technology like CRISPR-Cas9, which acts like a molecular scalpel so fine it can swap even single letters of DNA, has made a world of chimeric species possible. For some, splicing the DNA of extinct megafauna like dire wolves and mammoth into grey wolves or Asian elephants is an opportunity to resurrect a long-vanished world. But with synthetic biology we could potentially save the world of the present, intervening in the evolution of vulnerable species so they can survive changing climates and collapsing ecosystems. Plants could be edited to cope with drought, or to improve photosynthesis in crops. Microbes could be programmed to sense pollution and consume toxins, even the marine plastic that gluts the digestive tracts of seabirds. With assisted evolution, we might spare coral reef systems from collapse.
We shouldn’t approach this simply as a change in technology, but as an opportunity for a change of mind
The technical challenges of transgenic rescue are formidable. Every living creature’s traits are a combination of its genes and its environment. But it’s difficult to know precisely which combination of genes produces a particular set of traits; simply editing the genome also doesn’t change the fact that the complex ecological relationships that are crucial to shaping them may also be in flux. The ethical questions are perhaps greater still. Directing the evolution of other species looks a lot like playing God. In an age when international corporations have a stronger claim to legal personhood than living systems, and the genomes of commodified species like the broiler chicken have already been patented, the question of who decides which animals to alter and how can’t be ignored. For some, the best we can do for nature is to leave it alone.
But it’s an illusion to suppose that, at this point, our species can simply step back. The notion that ‘nature’ exists in a pure state is itself dubious, but if it were ever so, that time has long passed. ‘We’ are present in every molecule of anthropogenic carbon added to the atmosphere or the oceans, which will continue to shape the biosphere even if we were to immediately stop burning fossil fuels. Close to 50,000 species are at risk of extinction, according to the IUCN Red List. Not intervening may mean accepting loss on a frankly intolerable scale.
Four billion years of evolution prove that transformation is nature’s genius. But the scale and speed of humanity’s impacts suggest that even genius might need some assistance. If so, then we shouldn’t approach this simply as a change in technology, but as an opportunity for a change of mind. For millennia we have exported much of our evolutionary development to our cultures and technology as secondary inheritance systems, transmitting information and skills across time through social learning as well as our genes. Every new tool, from the stone knife to the internet, has altered the hand that wielded it. The question is, then, if we are to edit other species, can we do it ethically and wisely? And how should it also change us?
Corals are perhaps the best example of natural chimera. In appearance, they seem to straddle the boundary between plant and mineral; biologically, they are an ancient alliance between animal and microbe. Polyps, tiny soft-bodied creatures whose calcium carbonate secretions build vast reef conurbations, rely on microscopic zooxanthellae to provide nutrients and energy, as well as the photosynthetic pigments that paint reefs the colour of an acid dream.

Bleached coral. Courtesy the UN Ocean Image Bank
Corals show that an animal is more than its DNA; any living thing is a matrix of connections with other species (a point that critics of de-extinction often make: shorn of the web of living relations that sustained it, is a resurrected mammoth really a mammoth?) If ecology teaches us anything, it’s that being is a collaboration. But relationships can be fragile. Coral’s alliance is vulnerable to changes in temperature, and is steadily being unpicked as our broken climate spills heat into the oceans. Heat-nauseated polyps that vomit up their zooxanthellae will bleach and starve. Successive heatwaves have placed tropical coral reefs under intolerable strain. The current global bleaching event, which began in January 2023, has exposed more than 80 per cent of the world’s coral reefs to lethal temperatures. And corals are also the foundation of a densely populated environment. More than 4,000 fish species, as well as countless invertebrates, sharks, even humpback whales, live on or feed from tropical reefs. If we save corals, we will also rescue an ecosystem that supports around a quarter of life in the oceans; but with three mass bleaching events on the Great Barrier Reef in the past five years, time may be running out.
In 2015, the ecological geneticist Madeleine van Oppen, the marine biologist Ruth Gates and colleagues devised a suite of techniques to help coral keep pace with the rising heat. Their approach, which they call ‘assisted evolution’, includes ‘hardening’ (where heat-resistant traits are selectively bred into the coral), assisted gene flow (transplanting heat-tolerant corals to struggling reefs), and gene editing. Their idea was to step into the breach between coral polyps and their zooxanthellae, holding their alliance in place until we can bring down emissions and turn down the heat in the oceans.
Most current efforts to assist evolution look like a more conservative form of assisted evolution: domestication
Practical and straightforward, Van Oppen explained her motivation to me when I was researching and writing my book Nature’s Genius (2025). ‘I don’t want to control nature,’ she said, her Dutch accent barely inflected by years of living and working in Australia. ‘I just want to help it get over that hurdle.’
It may be that total control isn’t possible even if we wished it to be. Assisting coral evolution is not yet an exact science. The precise genetic recipe for heat-resistance hasn’t been mapped, and we don’t know which edits might entail unhappy trade-offs (heat-resistant coral may be less able to process nutrients, for instance). The risk of unintended consequences ought to be reason for caution. That said, most current efforts to assist evolution look a lot like a much older, more conservative form of assisted evolution: domestication. Just as early farmers cultivated crops and animals by selecting individuals with the most desirable characteristics to breed, most assisted evolution involves promoting conditions for coral themselves to share the genetic resources that might help the reef survive.
Some of the most promising projects involve collaborations with people who have lived alongside these ecosystems for millennia. The Yirrganydji have occupied Dawul Wuru – around 500 square kilometres of coastline in northern Queensland – for thousands of years. Kul-Bul, or Sea Country, stretches tens of kilometres from the coastline out to sea, including some of the worst heat-affected reef. In 2022, the Yirrganydji Land and Sea Rangers managed the transfer of heat-tolerant coral larva from neighbouring Gunggandji Sea Country to Dawul Wuru, combining traditional understanding of the use of marine resources with Van Oppen’s principles of assisted evolution. They are one of many such traditional owner groups working with coral scientists in coalitions of Western science and traditional ecological knowledge to help vulnerable coral clear the hurdle of rising temperatures.
Country is the foundation of Yirrganydji culture. It is the source of Dreaming, the living fund of story and landscape that sustains Indigenous cultures in Australia: like the coral dinoflagellates that feed the polyps, Dreaming nourishes connection. For the Yirrganydji Land and Sea Rangers, intervening in evolution means acting as partners with other species. Assisting evolution is, in the end, about forming alliances – with other species, and with different ways of knowing the living world.
Assisted evolution depends on the collaboration between reef scientists and traditional owners. But what if there was a truly empyrean technology – one that, effectively, could remake entire ecosystems with a single gesture?
Kevin Esvelt is a biologist who has shown this is possible – if we so choose. One day in 2013, Esvelt realised that he could solve endemic ecological problems by creating CRISPR edits that would pass from one generation to the next. Gene drives are elements in a genome whose heritable potential is enhanced. By exploiting this strain of bias in the system, Esvelt realised he could build a synthetic gene drive: a DNA-editing machine that, once added to the genome, would recur in each succeeding generation.
The power of a synthetic gene drive is difficult to overstate. Used for good, it could spread heat resistance through coral populations or immunity to anthropogenic diseases, or eradicate introduced predators; used for ill, it could become a terrifying biological weapon. A gene drive could be an extinction engine, giving the power of life or death over entire species to whoever wields it.
Aotearoa/New Zealand has one of the worst extinction records on Earth, due to the impact of introduced species
Esvelt couldn’t ignore the potential in gene drives, but nor could he ignore the risks. ‘The next day, I woke up in a cold sweat,’ he told me. He devised a series of safety measures that would limit the gene drive’s spread by scattering parts of its architecture across the genome, reducing the likelihood of a functioning gene drive appearing with each new generation. But a technical fix alone wouldn’t ensure that the fearsome technology would be used responsibly.
In 2014, Esvelt co-authored a paper that suggested gene drive could control invasive species in Aotearoa/New Zealand. For 85 million years, the frogs, bats, birds and lizards there lived in biological isolation. European settlers brought this to a brutal end as, first, rats, then, weasels and ferrets feasted on the defenceless local fauna. The country now has one of the worst extinction records on Earth, much of it due to the impact of introduced species. A targeted gene drive called ‘last litter’, where males are edited so that they cannot produce fertile daughters, could suppress the mustelid population in a matter of generations.
However, as he later acknowledged in a post on the MIT Media Lab website, Esvelt had not consulted Māori before he’d made this suggestion. The Māori conservationist Melanie Mark-Shadbolt’s observation that he had been ‘naive’ about the need for Māori co-governance on the matter of gene drives was, Esvelt conceded, ‘searingly true’. ‘I know as little of the local ecosystem as I do of the local politics,’ he said. ‘I cannot possibly evaluate the likely consequences.’ The decision whether to use a gene drive, he concluded, should include the community who would be most affected by it, informed by the principles that had governed their relationship with the land for generations. Esvelt learned from his mistake and invested time in consulting with different iwis, or tribes, to hear their views on gene drives. After speaking with Māori conservationists myself, I gained a clearer view of why this matters so much – and what the rest of the world might learn from the approach when weighing up interventions in nature.
Māori worldview rests on mātauranga, an intricate system of knowledge and values contoured to the ecosystem of Aotearoa/New Zealand. With a warm smile and an exuberant manner, the Māori conservationist Tame Malcolm explained to me how mātauranga gives rise to two principles that are the foundation of a Māori-centred approach to conservation. The first, he said, is whakawhanaungatanga. Like many Māori concepts, it has no direct translation to English, but the way Malcolm defined it was disarmingly simple: ‘Everything’s related.’ Whakawhanaungatanga is a deeply engrained process of forming relationships, between people, species and landscapes.
The second concept is whakapapa. Again, English lacks an equivalent. For Malcolm, it means genealogy. Whakapapa describes a family line, stretching back to creation, but that also includes the rivers and mountains an individual lives among. But Marcus-Rongowhitiao Shadbolt, another Māori conservationist, suggested a different translation to me. Rather than genealogy, Shadbolt thinks of whakapapa as a kind of taxonomy. ‘It’s a way of sorting the natural world,’ he said. But unlike the physiological commonalities of Linnean classification, on which Western animal and plant biology is based, whakapapa groups living things according to relationship.
‘Instead of putting two birds together because they are closely genetically related, in whakapapa you would put two birds together because their niches overlap,’ he explained. Or you might put together two organisms that, to Western science, would seem irreconcilably different.
Māori healers discovered that a balm made from the bones of beached whales could heal infected kauri trees
Once, Kauri, a coniferous tree, and Tohorā, the southern right whale, were siblings together on land. But where Kauri loved the soil and the sky, Tohorā longed for the oceans. Before Tohorā departed, they exchanged gifts: Tohorā dressed Kauri in his scaly skin, which became rough bark, and left his own skin so smooth he could glide between the waves. Kauri gave Tohorā oil to keep him warm and protect him from salt, and taught the whale to sing.

A kauri tree known as Tāne Mahuta in the Waipoua Forest, Aotearoa/New Zealand. Courtesy Wikipedia
‘Kauri said: “I’ll stay here and look after this place; you go off and explore, and then come back and tell me what you find,”’ Shadbolt explained. ‘Our stories tell us that Kauri and the whale have the closest relationship to one another.’
Remarkably, this turned out to be more than just a story. In the 1970s, kauri trees began to suffer from a fungal infection that attacked their root system. The infection spread in the early 2000s and is now killing large numbers of trees each year. ‘The first response of Māori was to go see what the whales were doing,’ Shadbolt said. Some said whales were beaching because they were drawn to help their stricken brothers. Then Māori healers, or tohunga, discovered that a balm made from the bones of beached whales could heal infected kauri trees.
This appeared to be a successful treatment for kauri dieback, said Shadbolt. ‘So, if you told someone that you could save the whales by using genes from a kauri tree, you’d have no opposition from Māori, because to them you’ve only gone back one generational step.’ If confirmed by scientific studies, it would not be the first time Māori knowledge has inspired treatment. Drawing on Māori experience of local plant ecology, researchers in 2019 showed that extracts from the kānuka plant inhibit the growth of the fungus-like organisms afflicting the kauri.
Shadbolt and Malcolm also helped me to see that any intervention we make in the evolution of other species has to be grounded in a deep and abiding sense of relationship. The questions Māori ask about gene editing revolve around whether it will enhance or diminish essential values like whakapapa. There’s no single Māori point of view on gene editing, Malcolm said. Every iwi could see the issues differently. Improving the resilience of a species or an ecosystem with synthetic biology could enhance kaitiakitanga, the principle of guardianship that makes Māori responsible to the rest of the living world; but the possibility that a gene drive could run out of control, despite Esvelt’s carefully engineered breaks in the system, risks undermining kaitiakitanga, making Māori irresponsible stewards. Some Māori might be sceptical of gene editing, because introducing a foreign gene to a species could diminish its whakapapa (even non-native species like rats and possums possess whakapapa, Malcolm explained); but it could also enhance whakapapa if it contributed to a species’ long-term wellbeing or the functioning of an ecosystem.
Esvelt has engaged in lengthy conversations with Māori about synthetic gene drives, and for now at least there’s no consensus as to whether they represent a solution to Aotearoa/New Zealand’s beleaguered ecosystem. The government is exercising caution, and the technology is highly regulated. But there remains keen interest in gene drives; if they are ever used, then whakapapa – which supplants species boundaries with the bonds of ecological relationships – tells us something vital about how we should approach synthetic biology.
All living things have whakapapa; in the case of other animals, it is the evolved relationship between body, behaviour, and place that fits the animal to its environment and the other creatures it lives with or depends upon. It’s unfortunately true that no animal can give their permission to edit their genome; there is no parliament of species to provide a mandate. But we can, as it were, enquire after its whakapapa – the lines of inheritance and relation that connect it to the larger web of life. This is not to say that other cultures ought to appropriate Māori beliefs, but that the same question – would this enhance or diminish connection between species? – should apply.
Possessed of such tremendous power, what does it mean to be human?
Editing cattle to withstand heat may fail the test, if our motivation is simply to keep eating beef. This would surely diminish our relationship in a wider sense, perpetuating a scenario of profound ecological imbalance, where livestock make up two-thirds of mammalian biomass and leading to further destruction of forest ecosystems to make way for pasture. But some changes foster relation: editing American chestnut trees with a gene derived from wheat allows them to coexist with a fungal pathogen that has nearly wiped out the entire chestnut population. (Although transgenic chestnuts perform poorly in the wild – the wheat gene, which produces an enzyme that suppresses the fungus, also reduces the trees’ ability to withstand drought – illustrating the profound difficulties of successfully editing a species’ genome.) Using gene editing to help tropical corals withstand bleaching would also sustain the thousands of species that co-exist with reefs. Even if this required using genetic material from an entirely different species, the imposition on coral genomes would be felt by countless other species as a continuation, a furtherance of life.
To establish an animal’s whakapapa involves tracing its relationship back to the Atua – the gods – that rule the place where it lives. Rather than posing humans as gods, a Māori-centred approach invites us to recognise that we, along with all living things, have inherited a thread of the divine. This is the fundamental question presented by gene drives, and by gene-editing technologies in general: possessed of such tremendous power, what does it mean to be human?
In 1968, the writer Stewart Brand – who has since become a supporter of de-extinction genetic technologies – proclaimed: ‘We are as gods and might as well get good at it.’ But what does it mean to ‘get better’ at playing God? The sense of ourselves as a species apart, gilded by technological prowess, is the seed from which much of the destruction of biodiversity has sprung. Instead, we might look again to the Lion-Man, in whose form human and animal seamlessly blend into one another. Our ancestors knew that to reimagine other beings is to also reimagine ourselves. If we are to intervene in the evolution of other species, we should do so recognising the filament of sacred life running through all things, and ask ourselves one simple question: would this draw tighter the threads that stitch us into the weave of the living world?