‘Why don’t you use fat?’
I stared at Keith, not quite sure whether he was serious or just kidding. Did he really think we could use fat to regenerate the heart?
I had joined Keith March’s research laboratory at Indiana University as a postdoctoral fellow in the summer of 2001. At the time, his group was trying to improve upon stents, small mesh tubes that can be placed inside blocked coronary arteries to keep them open, restoring an adequate supply of blood and oxygen to the heart. But even the best stents were no cure for heart tissue that had already been irreversibly damaged by a heart attack. The wave of the future, I felt, was the newly emerging field of cardiovascular regeneration, the idea of using stem cells to repair the heart and grow new blood vessels.
Yet when Keith suggested I use fat to generate those cells, I thought he was making an inside joke. We were both overweight and often made fun of ourselves. And the history of fat cures was rife with superstition and myth. For centuries, people had believed that rubbing one’s arms and legs with balms made out of human fat could cure broken bones, crippled limbs and joint pains. Societal mores prevented the dissection of human bodies for the purpose of removing human fluids or tissues, but these rules didn’t apply to executed criminals, especially when there were no family members to claim the body. Until the mid-18th century, this presented a lucrative opportunity for a group of social outcasts: executioners, who became expert extractors, with a skill-set and knowledge of anatomy that often surpassed that of academic physicians. In her book, Defiled Trades and Social Outcasts (2000), the historian Kathy Stuart from the University of California, Davis, gives a gripping account of the work and lives of executioners. Some executioners even started their own medical practices, selling products such as human fat themselves.
Today we understand that rubbing human fat on one’s limbs lacks any medical benefit – yet there was Keith suggesting that fat could be rediscovered as a therapeutic source of material to regenerate the heart. I took him seriously only after he showed me a paper written in 2001 by the cell biologist Patricia Zuk and her colleagues at the Regenerative Bioengineering and Repair Lab then run by Marc Hedrick at the University of California, Los Angeles (UCLA). Analysing fat obtained through liposuction, the team of researchers and plastic surgeons had discovered an abundance of adult stem cells! It was an extraordinary find: it was in part the scarcity of adult stem cells, after all, that had stymied organ regeneration efforts in the past.
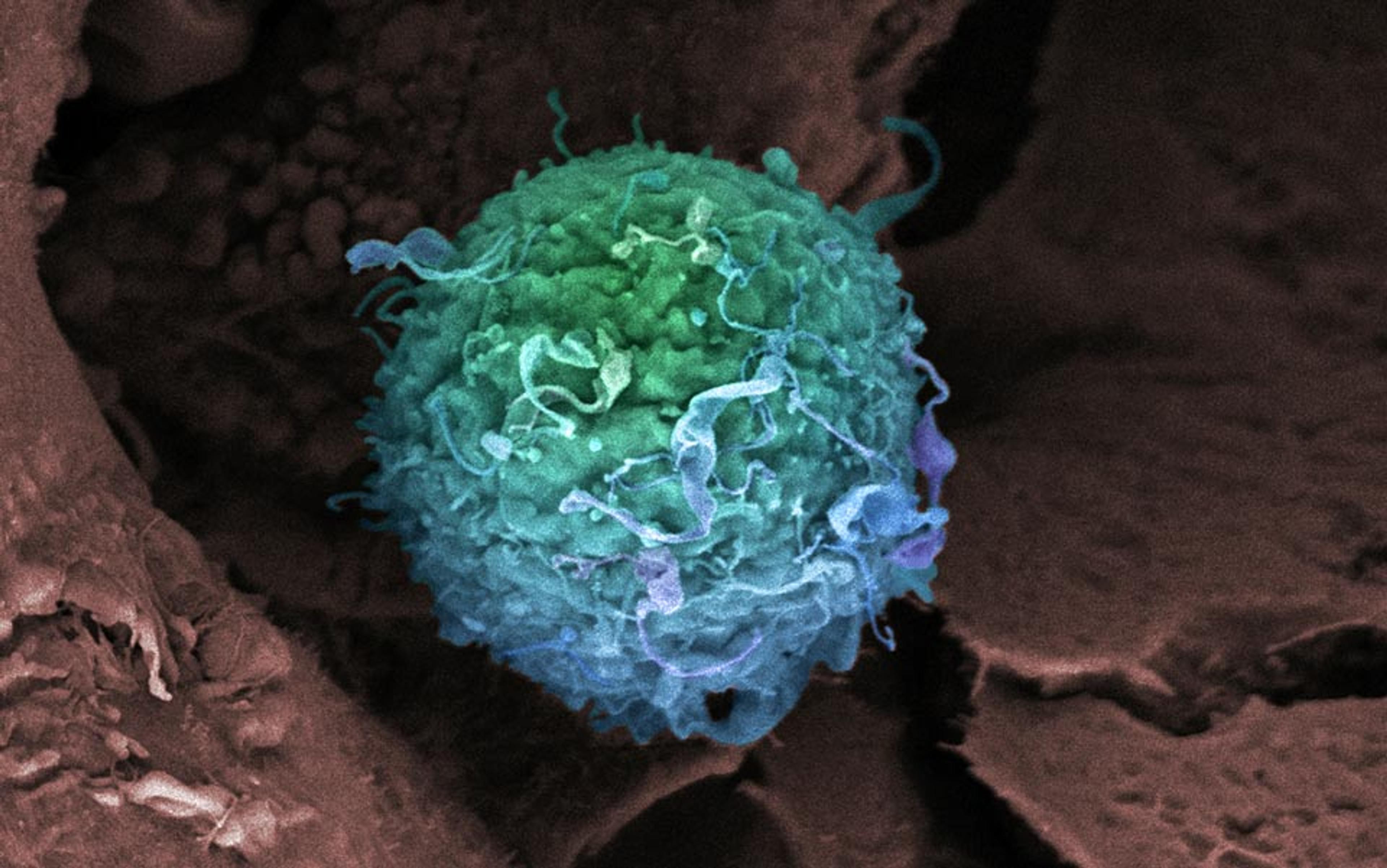
Stem cells can be tapped as engines of regeneration because they have not yet committed to a specific cell fate. They are so pliable that they can be converted into a variety of prized cell types, such as neurons or beating heart cells, if exposed to the proper chemical and environmental cues in the lab. That makes them ideally suited to repair or regenerate diseased organs and tissues. For example, if the heart is damaged and scarred by a severe heart attack, stem cells could be used to replace the scar tissue with beating heart muscle cells (cardiomyocytes).
In 2001, researchers had access to two types of human stem cells: embryonic stem cells and adult stem cells. The advantage of using embryonic stem cells is that they can be converted into every major cell type required for organ or tissue regeneration: liver cells, neurons, or cardiomyocytes, to name just a few.
Yet use of human embryonic stem cells, generally derived from leftover fertilised eggs culled en masse from fertilisation clinics, has long been controversial in the US, even compared by some religious groups to murder. In response, in August 2001 the government restricted use to the few human lines of embryonic stem cells already cultured in labs.
There were scientific obstacles, too. Embryonic stem cells can be expanded indefinitely because they contain high levels of the anti-ageing protein called telomerase, the same protein that helps confer immortality onto cancer cells. When embryonic stem cells are converted into mature cells, they lose their cancer-like immortality. But there is always a risk that a tiny fraction of immortal stem cells might remain undetected and could potentially form a tumour. Furthermore, all regenerative cells derived from embryonic stem cells carry the genetic signature of the original fertilised egg – foreign donor tissue. Therefore, if a patient receives a new organ or tissue engineered from human embryonic stem cells, they might require immune-suppressive therapy; such therapy prevents rejection but opens the door to infectious disease.
Adult stem cells have some advantages. They are not immortal and thus less likely to form tumours. They don’t come from embryos and lack the political baggage. And they can be taken from the patient him or herself, thus reducing the risks of immune rejection.
But adult stem cells also fall short. They are more mature than their embryonic counterparts, and thus constrained in their capacity to convert into other cell types. For instance, blood-forming adult stem cells from the bone marrow can be converted into red blood cells and white blood cells, but it is not possible to reliably derive cardiomyocytes from them. Mesenchymal stem cells are another form of adult stem cells that can be found in the bone marrow. They can give rise to bone, fat and cartilage, but we have little evidence that they consistently form cardiomyocytes. Another major hurdle in adult stem cell therapy is that our bodies contain only a finite quantity. Every time we expropriate a reservoir of adult stem cells for new therapies, we run the risk of compromising their original purpose. Extraction of large quantities of adult stem cells from an individual’s bone marrow to treat heart disease, for instance, can exhaust the marrow’s ability to fulfill its primary function, producing blood cells.
The discovery at the UCLA lab in 2001 seemed poised to change everything. If something as simple as tapping into our fat depots solved the problem of scarcity, then we wouldn’t have to worry about the risk of extracting precious bone marrow or other, even rarer, stem cell reservoirs. It seemed so simple: enormous quantities of fat stem cells might be residing right underneath the surface of our skin.
But nothing is ever as simple as it seems.
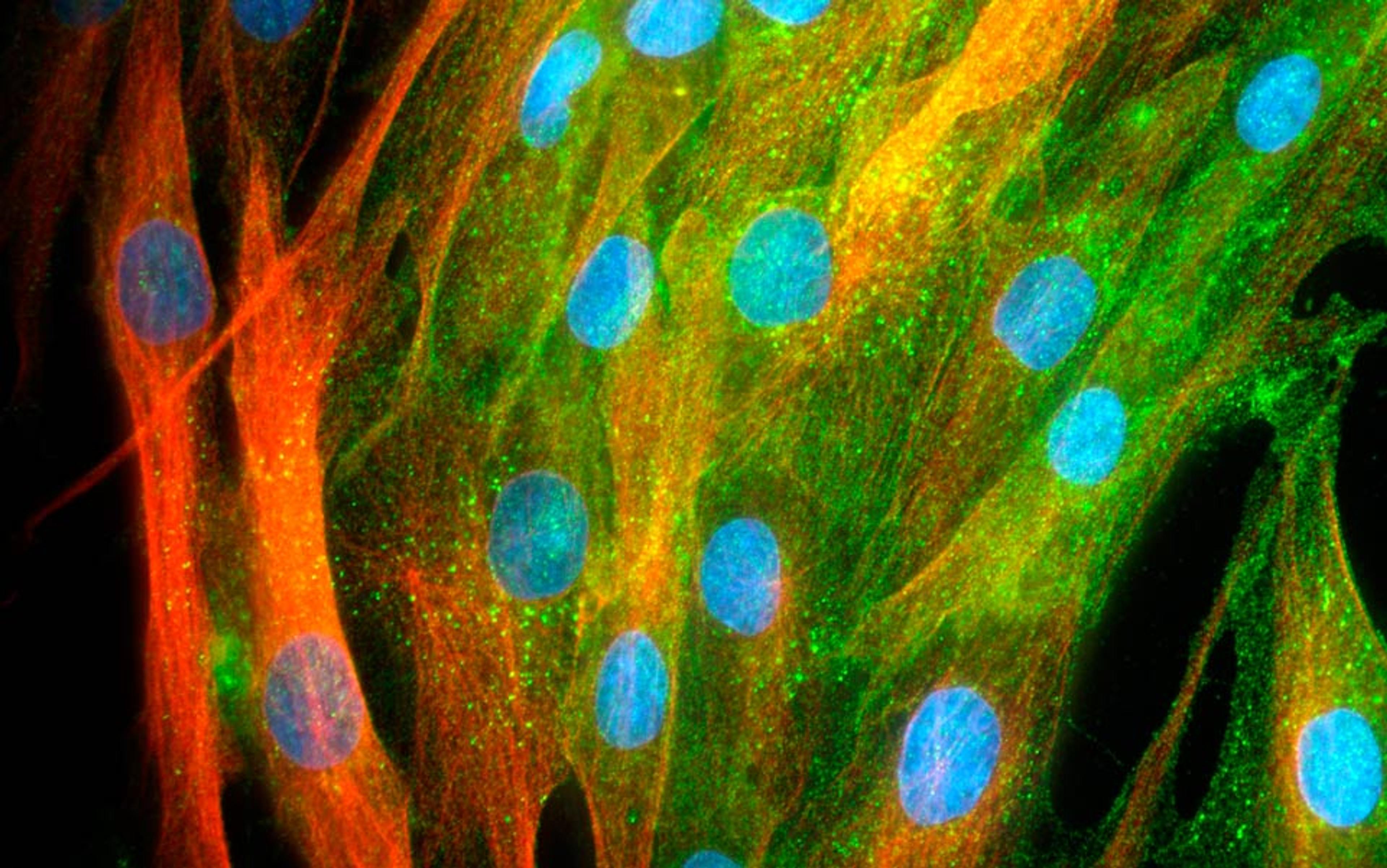
To do their research, the UCLA team had carefully parsed fresh liposuction samples from patients. They first removed the adipocytes – mature fat cells chock-full of fat droplets. The separation step was straightforward because fat is lighter than water and thus floats to the top. After discarding the mature fat, they focused on the remaining cells, which they referred to as ‘processed lipoaspirate’ (PLA). Analysing the PLA, they found a variety of cell types: immature fat cells, blood vessel-forming skin (or endothelial) cells, and scar-forming fat cells.
The researchers decided to seek stem cells hidden somewhere within this PLA mix. Their rationale was intriguing. If the PLA contained immature fat cells that had not yet soaked up fat droplets, perhaps those cells could be coaxed into becoming other cell types – in other words, perhaps the immature cells were stem cells.
To test the hypothesis, they exposed the PLA to the same proteins and growth factors already identified as cues to convert bone marrow-derived mesenchymal stem cells into bone and cartilage. The strategy worked: using those same growth factors, PLA cells from human fat could indeed be converted into bone and cartilage. Not only that but, unlike ordinary adult stem cells, one could grow the PLA cells in abundance in a culture dish. After a few weeks, a million PLA cells could readily be turned into billions of cells, more than enough to engineer tissues. A single liposuction aspirate contained enough of these cells to create layers of bone and cartilage, which might eventually treat patients who suffered from bone fractures, osteoporosis or chronic arthritis. The whole idea of converting worthless fat into valuable cartilage and bone for tissue regeneration had an alchemical ring to it, but the scientific data in the paper were solid.
Once I understood the implications, I became so excited I immediately called Keith March and told him I would work on this project. Converting fat into bone or cartilage was great for orthopedic purposes, but we were cardiologists. My goal was to convert fat stem cells from liposuction aspirates into cardiovascular cells, such as cardiomyocytes or endothelial cells that form the lining of all blood vessels. We would then be able to use the cells to restore heart function or grow new blood vessels in patients with heart disease. By the end of 2001, we had assembled a team of researchers at Indiana University to help us carry out the experiments. Keith also arranged for a steady supply of liposuction fat from a local plastic surgeon.
When I placed these cells next to cardiac cells of a rat, they began to beat in sync. I could hardly contain my excitement
Using the methods that the UCLA team had pioneered, we obtained several million PLA cells that could be expanded in a culture dish. Then came the more challenging part: converting fat cells into precursors for treating heart disease, including cardiomyocytes to regenerate heart muscle and endothelial cells to repair or grow blood vessels.
First we had to develop recipes for the conversion process, building on methods other stem cell researchers had used to generate cardiomyocytes and endothelial cells from either embryonic or adult stem cells. Most of the recipes required weeks of exposure before we could know whether or not they worked. I remember being quite impatient on some days, even removing the cultured cells from the cell incubator every few hours, hoping to somehow glimpse the conversion taking place in real time. But the PLA cells paid no heed to my impatience – in fact, the recipes seemed to be busts. I spent much of 2002 trying to tweak the formula, changing the number of cells we plated and modifying the growth factor combinations.
Eventually, in a handful of experiments, a few fat-derived cells began to contract and beat rhythmically, just like real cardiac cells. When I placed these cells next to cardiac cells of a rat, they even began to beat in sync. I could hardly contain my excitement; after months of trying, we finally saw proof of the potential. Unfortunately, when we tried to repeat the experiment in the subsequent weeks, we did not see a single fat-tissue derived cell that looked like a beating heart cell. My attempts to convert the fat-derived cells into blood vessel endothelial cells were also quite disappointing. In 2002, the UCLA team published another, more comprehensive paper confirming their earlier findings that human fat contained adult stem cells. Other labs confirmed the findings as well. But all that evidence only made it more frustrating for us – we were getting nowhere.
In the beginning of 2003, we were close to giving up on the project when we decided to pursue one final line of inquiry: even if fat’s repertoire was too limited to produce cardiac cells, I hypothesised, perhaps it could still help repair the heart by an alternate path. I based my suspicion on reports that adult bone marrow mesenchymal stem cells released growth factors that nourished and accelerated the regeneration of neighbouring cells. Researchers were increasingly calling these stem cells ‘stromal cells’, from the Latin ‘stroma’ for mattress or covering, to signify their support function.
Perhaps PLA cells also had a support function that could be used for regenerative therapies? Instead of expending all our efforts on trying to convert PLA cells into endothelial cells, perhaps we should have been growing PLA cells side by side with endothelial cells and studying what occurred.
In the spring of 2003, we conducted those experiments. As expected, human endothelial cells placed on special gels self-organised into networks of thin tubes. But when we added PLA cells to the gels, the growth of the blood-vessel-like tubes increased several-fold. A mere glance through the microscope revealed a day and night difference: instead of scrawny tubes formed by the endothelial cells alone, the PLA cells created thick structures that looked like true blood vessels.
The next step was finding the growth factors that produced this tremendous effect. Analysing the PLA genes and proteins they produced, we found not one, but a multitude of factors that promoted survival, regeneration and growth of blood vessels. Synergy between all the factors likely explained why they were so effective. These growth factors not only activated the growth of endothelial cells, they also made endothelial cells more resilient to stress. In the aftermath of this work, I decided to rename PLA cells ‘adipose stromal cells’ (ASCs), to emphasise the fact that it was their ‘stromal’ or caretaker function that aided in their ability to form blood vessels.
Especially important was the discovery that ASCs did not just blindly churn out growth factors. Their productivity was regulated by the cell’s ability to sense oxygen. When placed in a low-oxygen environment, the ASCs doubled or tripled production of factors necessary for the growth of blood vessels. This innate ability made ASCs a very attractive option for treating cardiovascular disease. The heart and limb muscle tissues of patients with blood vessel blockages suffer from low oxygen levels; implanting ASCs that released therapeutic molecules based on oxygen levels seemed almost too good to be true.
To explore the therapeutic value, we cut off blood supply to the lower parts of the legs of experimental mice. Half the mice then received an injection of human ASCs into the leg muscle. We observed an astonishing recovery of blood flow through new blood vessels in ASC-treated mice, but not our controls.
We had set out hoping to convert fat stem cells into cardiovascular cells, but instead, we had identified what seemed like a potent new tool for regenerating the vascular system and healing the heart.
Keith March and I would have pursued this work even if we’d been thin. But as overweight men immersed in a culture of fat-shaming, where our heft was often seen as a sign of moral depravity and gluttony, we derived great pleasure from showing that fat was not pure evil and could be put to good use. Our paper was accepted for publication in the journal Circulation in January of 2004, and the hoopla began. Newspapers and websites were just as intrigued by the idea of transmuting fat for cardiovascular purposes as Keith and I had been. I even began receiving phone calls at home, either from journalists or patients who wanted to know if they should undergo a liposuction procedure to extract stem cells for their heart. For some reason, people who were most adept at finding my home phone number were also the least adept at adhering to time-zone etiquette. I occasionally found myself answering the phone in the middle of the night and having to explain our work.
‘No, we were not saying that it is good to be fat. In fact, we do even know whether ASCs from patients with obesity or diabetes are as potent as those of normal weight subjects.’
‘No, not all of fat consists of stem cells, maybe just a tiny fraction.’
‘No, please do not gain weight just to create a cache of stem cells.’
It is difficult enough to give cogent answers during regular working hours, but it is much harder when you are woken up at 1am and the voice on the other end of the line greets you with ‘Are you the fat scientist?’
Since the UCLA lab published its work in 2001, the field of adipose stem cell biology has blossomed. The major push has been to translate the laboratory findings into clinical studies – and treatments. Today, cardiovascular patients in studies are receiving injections of their own ASCs after undergoing a liposuction. The hope is that the ASCs will either grow blood vessels or improve heart function.
Yet these treatments, too, might carry risks. Even though ASCs come from the patient him- or herself, they are still ‘foreign’ when injected into the heart, in the sense that they don’t belong there. They might not initiate a classic immunologic rejection, but if the injected cells do not find the proper scaffolds, they will probably die – and the cell death could provoke a damaging inflammatory response. What’s more, there is little evidence that ASCs survive in the body for more than a week; treatments in heart patients can thus require repeated cell injections using catheters that have to be manoeuvered into the heart or the coronary arteries – and such repeated injections are not without risk. We have so much to learn, in fact, that clinical trials where cells are squirted into the human heart could be premature.
Why, then, the rush? The answer can be found in a famous Sufi story about the wise fool Mulla Nasruddin, who was looking for a lost key in the street when a friendly neighbour stopped to help him. They were both on their knees searching for quite some time. Frustrated, the neighbour asked Nasruddin where exactly he had lost his key so that they could retrace his steps. Nasruddin responded that he had lost the key in the house.
‘Then why are we out here looking for it on the street,’ asked the neighbour with a bewildered look on his face.
‘That is simple,’ answered Nasruddin cheerfully. ‘There is more light out on the street than in my dark house!’
From my perspective, the ongoing cardiovascular cell therapy trials could be much the same – convenient. Injecting regenerative cells through a catheter into the heart is less invasive than another approach – surgically implanting scaffolds that retain the cells within the body, which could be more enduring. Refining the technique further by identifying ASCs with the most regenerative power would require more work still.
Patients suffering from chronic illnesses, disillusioned by academic medicine, become the perfect victims for predatory abuse
Some researchers hope that the cruder approach of current trials will be so successful that more nuanced work need not be done up-front. But if the trials fail, then detailed studies on the regenerative mechanisms of ASCs will be the only way to yield fruit: how do we identify the most potent ASCs? How can we deliver cells to targeted areas of the heart that need them most? How can we make sure they are retained and integrated in the tissue so that they can continuously release the right proteins? With this kind of targeted therapy, we should be able to salvage much of the tissue at risk of dying after blood flow to the heart is cut off.
But despite the need for research, there is a rush to use – not just to perform clinical studies but also to market unproven therapies to patients. I have come across a number of websites advertising liposuction ‘stem cells’ to treat or even cure heart, lung and neurological disease. Yet there is no mention of the fact that liposuction material is a complex conglomerate of multiple cell types. There is no standardised protocol for cell isolation and characterisation, so it is not always clear what cells are being infused back into the patients. Some of these cell preparations could even induce inflammation or scarring, processes very harmful to a heart that has just suffered a heart attack. Patients who are suffering from chronic illnesses and are disillusioned by the therapies that academic medicine has to offer become the perfect victims for such predatory abuse.
The irony is that the less effort such clinics spend on processing and characterising the cells they are injecting into patients, the more likely they are to get away with it. The US Food and Drug Administration (FDA) makes exemptions for treatment with one’s own cells, as long as modifications are no more than minor and cells are re-injected during the same procedure. If a clinic offers to perform a liposuction on a patient, extracts the cells and immediately infuses them into the heart or lungs, it might be able to apply for an exemption from the FDA.
The discovery of regenerative cells within our fat has opened up new doors. As adult stem cells, they can be converted into tissues such as bone and cartilage and might provide long-sought relief for debilitating diseases such as chronic joint pain. As stromal cells, they are able to build and regenerate blood vessels, and could provide relief for millions of patients affected by poor blood flow to their vital organs. With scientists starting to engineer organs such as the heart, lungs, pancreas and liver from scratch, they are realising that ensuring blood supply to newly engineered organs is critical. The ability of cells derived from fat to grow blood vessels might make them central players in the future of organ engineering.
Discovering the regenerative power of human fat also begs a bigger question: how much more therapeutic potential resides within our bodies, just waiting to be discovered by scientists of the future? Stem cell research and regenerative medicine are providing humankind with an unprecedented array of opportunities to realise the age-old human quest for rejuvenation and longevity. But just like our predecessors – those physicians of centuries past who rubbed patients’ limbs with the fat of the dead – we can be seduced by false hopes and hypes. Stem cell biology has had more than its share of setbacks, often because it inspires dreams and promises that outpace the capacity of the science. Yet, propelled by those dreams and gigantic aspirations, we should be able to overcome obstacles, turn our back on false science, and engineer the transformative medicine to come.
References and links to the research mentioned above are available here
Disclosure: Jalees Rehman is a co-inventor on a patent to use fat-tissue-derived cells for therapeutic purposes.