Suppose you wanted to preserve a room completely unchanged. Picture an Egyptian tomb, or perhaps a storage vault for fragile artefacts. Suppose that your maintenance budget is effectively zero. How should you arrange things? Is it better to seal up your chamber against every possible disturbance? Or perhaps you make a provision for cleaners to come in whenever resources permit. They might not be able to do much. They might knock things over. But still, perhaps they’re better than nothing – a fragile, fallible defence against the forces of entropy. It is said that if you want to survive, standing still is not an option. Is that always true?
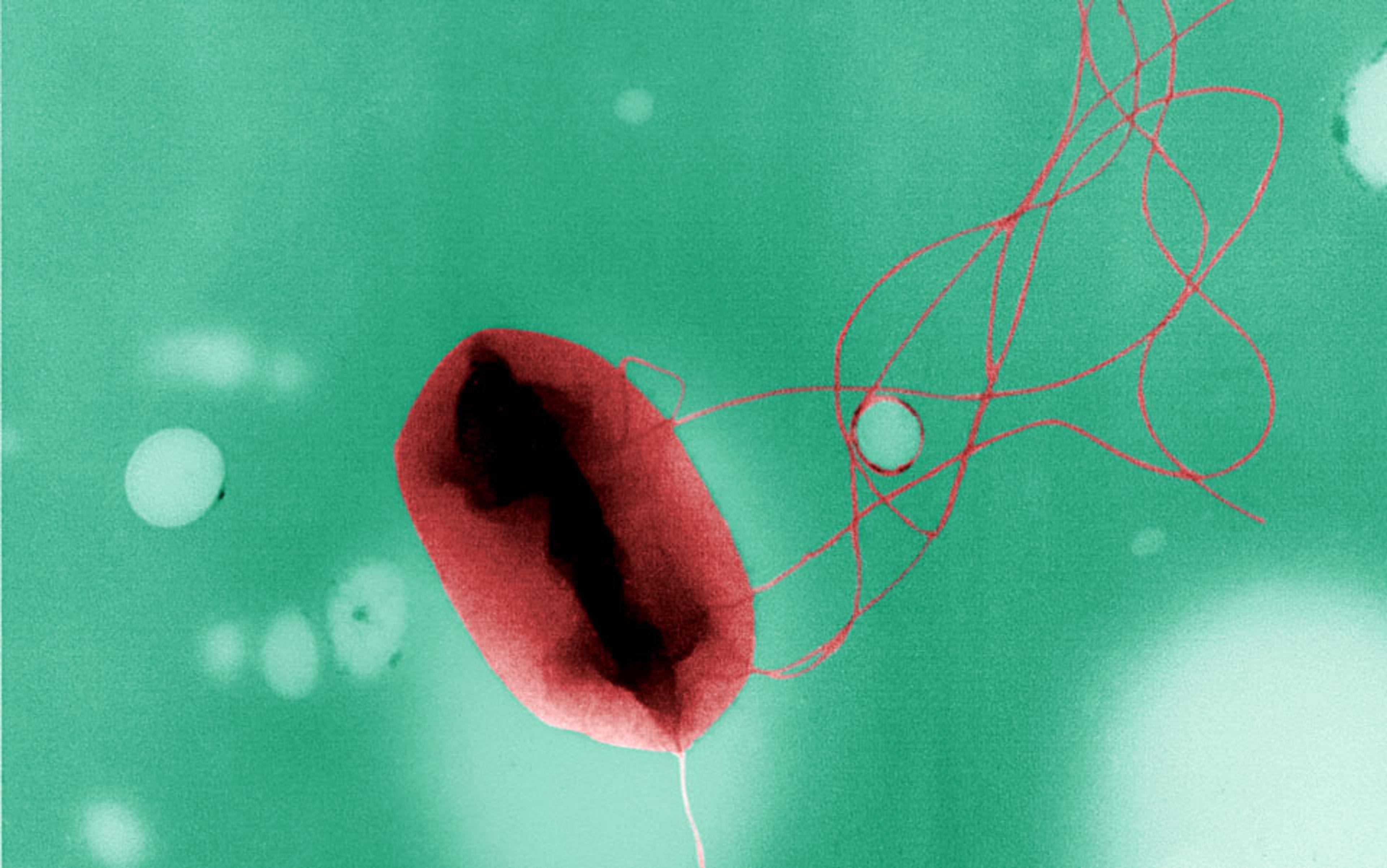
One evening at the British Antarctic Survey in Cambridge, a post-doctoral researcher named Charles Cockell placed another Petri dish on the laboratory worktop in front of him. He opened the circular lid and spread an aliquot of bacteria on the amber-coloured agar. His movements were deft, like a worker on a production line. He had performed the same routine countless times. Using a permanent marker, he wrote the species name (Chroococcidiopsis), the date, and his initials on the lid, then sealed it shut.
Each sample was to be exposed to harmful radiation. Cockell’s task was to assess the resilience of the bacteria, tallying up the living and the dead. That was the plan, anyway. But as the weariness of a long day crept in, he made a mistake. A Petri dish got misplaced. The agar cracked as it dried.
Ten years passed.
In 2013, after years of searching for hardy bacteria underneath Arctic and Antarctic rocks, Cockell unpacked his boxes ready for his new position as professor of astrobiology at the University of Edinburgh. Hidden in a forgotten drawer, he re-discovered this same errant sample. And so he asked himself what any biologist would ask: had they survived?
In the wild, this strain of Chroococcidiopsis lives in the rocks of the Negev Desert in Israel. Infrequent rains and searing sun mean that water is available for around 50 hours a year only. These single-celled organisms, known as cyanobacteria, have evolved to tolerate extremes of water deprivation. By drying themselves down, replacing water with sugars and amino acids, and concealing themselves within a tough shell, they batten down their tiny hatches for the drought ahead. They lay dormant, waiting for conditions to improve.
Bacteria not too dissimilar to this have resumed growth after as much as a century of desiccation, sealed safely within herbarium specimens. But other species have been alleged to survive longer – much longer. Stories of their miraculous revivals dot the scientific literature. Concealed from the outside world (within salt crystals, or in a bee fossilised in amber, or a mammoth’s frozen intestines), their periods of dormancy are said to span millennia, even millions of years.
Such claims are controversial. The reproductive scientist Malcolm Potts complained: ‘I find these arguments to be rooted in faith (“I believe”, “I don’t believe”) rather than in rigorous, factual, deductive, reasoning. Such dialogue contributes little to our knowledge of cell longevity.’ Is he right? It’s difficult to say. The problem is, we are analysing only the final chapter of an epic tome on microbial survival over long timescales. The preceding chapters are missing.
Potts went on to declare that the necessary experiments for investigating microbes over geological time periods are ‘not feasible’. Cockell can’t change that, but he can do the next best thing. After finding that his cyanobacteria did survive, germinating into small ‘green blobs’ after a month of waiting, he has extended this 10-year accident into the longest microbiology study ever planned. Started on 1 July 2014, the ‘500-year experiment’ will monitor bacterial dormancy over century timescales, providing scientists – present and future – with a unique portal into this little-known world. To understand the relative immortality of microbes, Cockell has put his own mortality aside.
For almost as long as we have known about micro-organisms, we have known about dormancy. In 1702, the Dutch biologist Antonie van Leeuwenhoek collected some dried ‘animalcules’ from a nearby gutter and added water. Peering through his handmade microscope, he observed that ‘they began to extend their bodies and in half an hour at least 100 of them were swimming about the glass’.
Aged 70, van Leeuwenhoek had just discovered the dormant states of rotifers – small, wheel-shaped animals that can be found in many transient freshwater habitats. When conditions become too Spartan, these humble organisms contract into dry, oval-shaped husks in order to survive.
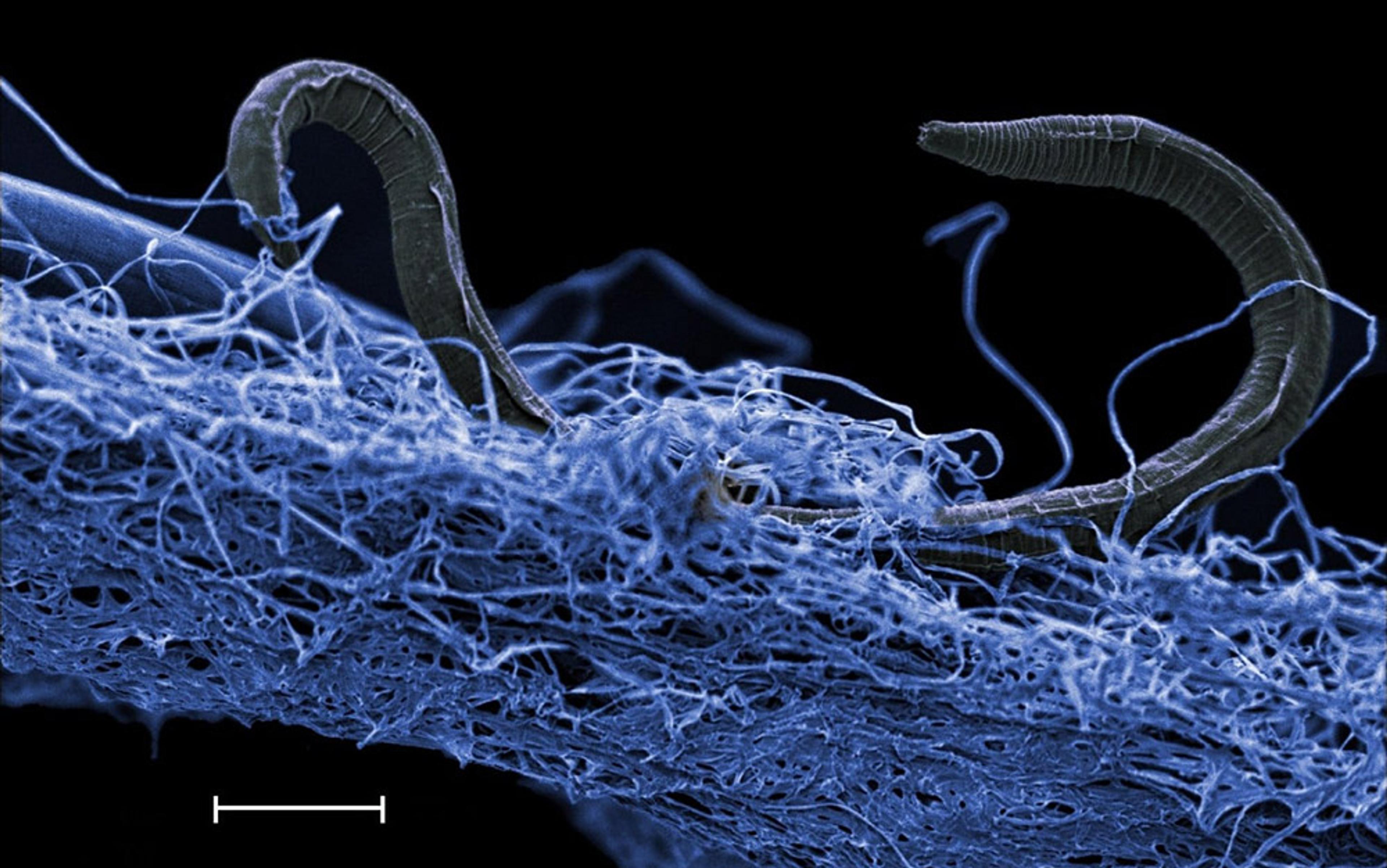
Van Leeuwenhoek continued his investigations up until his death on 26 August 1723, aged 90. But few of his contemporaries were interested in microbes, and his work fell into its own peculiar state of dormancy. It was only after several decades of neglect that a cadre of biologists started to revisit the topic of microscopic stasis, focusing first on the nematode worms that blighted wheat grains.
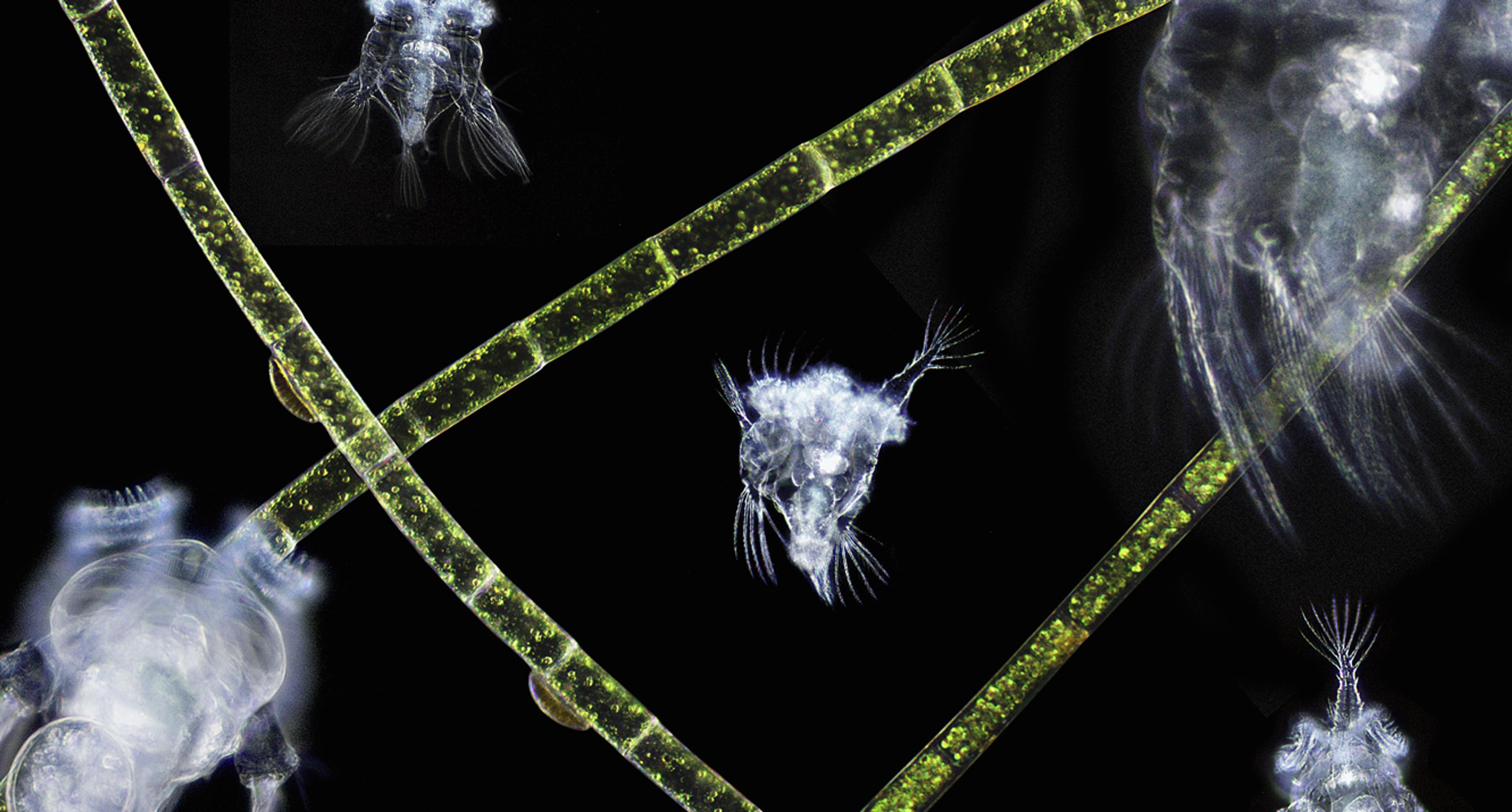
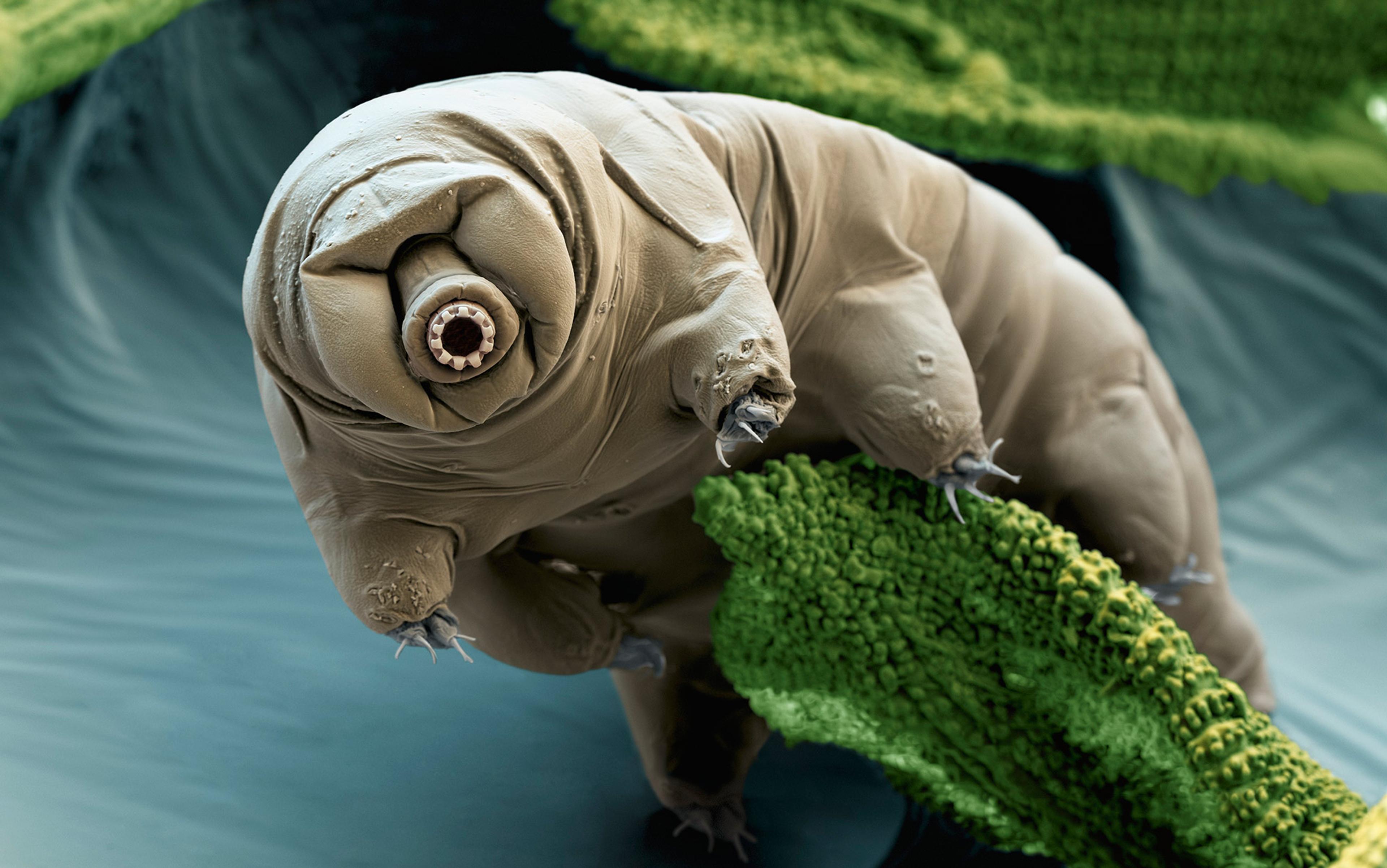
In the years that followed, a menagerie of minutiae – bacteria, tardigrades (tiny animals, also known as water bears, with a protrusible cannon for a face and six spiky legs), brine shrimp, and yeast cells – have been found to depend upon spells of stasis. Collectively known as anhydrobiotes, each can extend an otherwise short lifespan into an indeterminately long one. Despite their differences in appearance and scattered positions on the tree of life, their method of survival is remarkably similar: protect the DNA; protect the proteins that can repair the DNA.
the genetic instructions are placed in a many-walled fortress. The gates are locked, the only key is the return of favourable conditions
Water is essential for life, and yet anhydrobiotes appear to get by without it. How? According to the ‘water replacement hypothesis’, they exchange their cellular fluid for sugars such as sucrose and trehalose. The result is a glass-like substance that not only retains the cell’s shape on rehydration, but also slows down a lot of unwanted chemical reactions. With this scaffolding in place, they reduce the fires of their metabolisms to embers, conserving their energy like a ground squirrel within its winter den, waiting for conditions to improve. They keep things ticking over.
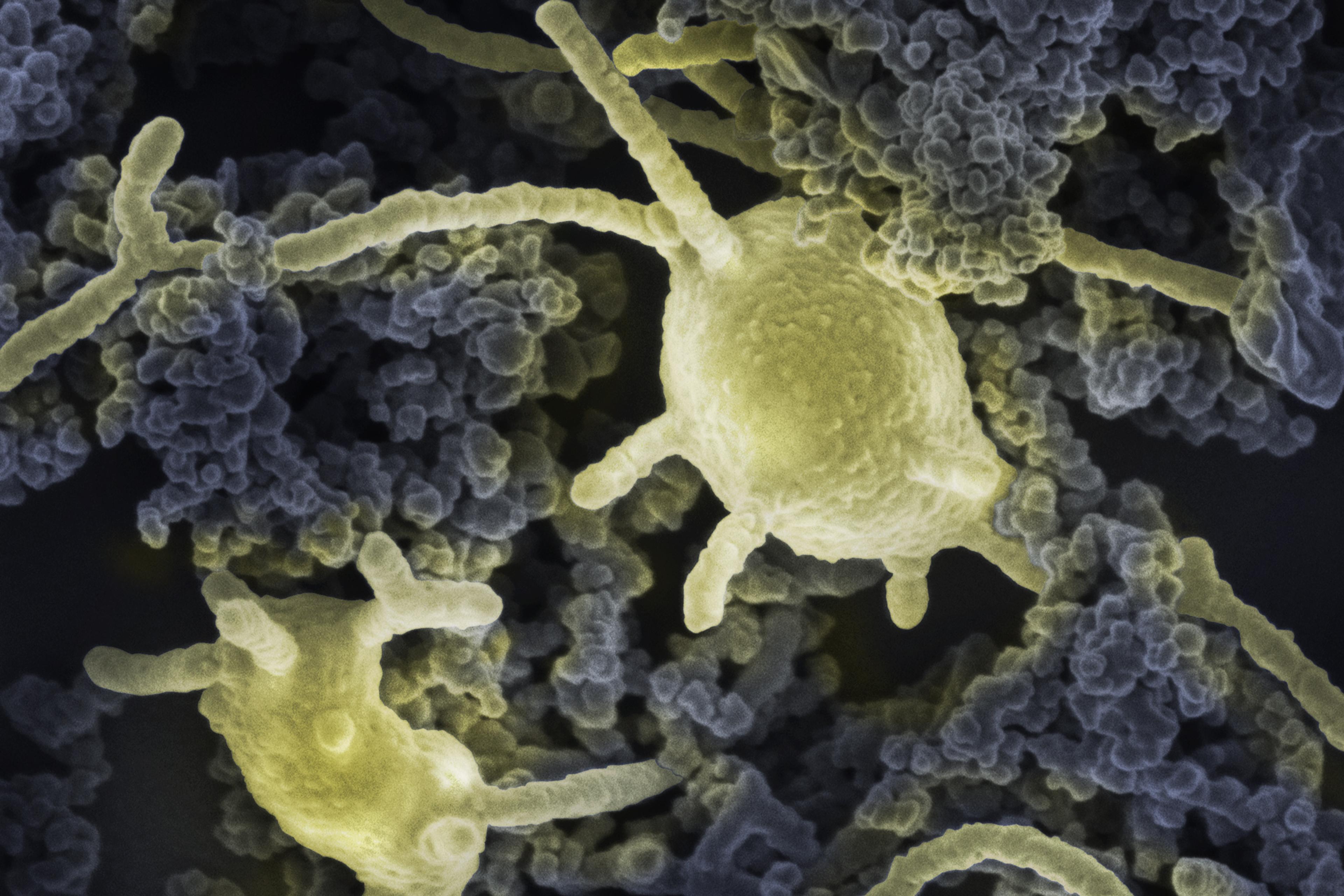
That’s how cyanobacteria seem to pull off the trick. But other organisms favour a more radical approach. Instead of enclosing the entire cell, they form tough endospores – multilayered cocoons that envelop only their vital molecular machinery. Unable to switch to a power-saving mode like the cyanobacteria, they are forced to turn off their metabolisms entirely. ‘The spore is a very specialised state of dormancy,’ says Cockell. ‘The DNA is wrapped in particular acids and proteins, and there are numerous coats that protect the DNA from the environment.’ In other words, the genetic instructions are placed within a many-walled fortress. The gates are locked, and the only key is the return of favourable conditions.
Such precautions are necessary. Both UV and background radiation cause DNA to mutate and break apart, producing highly damaging molecules as a by-product. These so-called reactive oxygen species then ricochet around the cell’s innards, continuing the process of decay. In dried-down cells, with their cellular defences still functioning on a low-energy cycle, such molecular errors can be repaired, albeit slowly. But in the absence of any metabolic activity at all, endospores can repair DNA only once they rehydrate. In the meantime, they are utterly dependent on their cocoons for insulation against these external threats.
Since their discovery, dormant microbes have been exposed to myriad experimental treatments, stretching their fortifications until they collapse. They’ve been launched into space, irradiated, pressurised, frozen and burnt. Endospores have even been dropped into hydrogen peroxide, only to find that they convert the harmful, reactive molecules into harmless water and an oxygen floatation device. ‘[They are] converting something damaging into something beneficial,’ observes Andrzej Paszczynski, a biochemist at the University of Idaho, whose student discovered this last survival trait. ‘That is what life is able to do over millions of years of evolution.’
But to elucidate their tricks of survival in real time, there is no surrogate for simply placing samples in a box and leaving them there.
The apparatus is simple: it looks a little like a 19th-century chemistry set. The samples, contained within an assortment of glass vials in a wooden case, are spore-forming bacteria or dried-down cyanobacteria. Each population will be analysed for signs of life and death after predetermined sampling points.
In fact, there are two boxes – one held at Edinburgh and the other at the Natural History Museum in London. Inside each are 400 glass vials, separated onto two shelves. Each vial contains one of two species: the cyanobacteria Chroococcidiopsis and another species of bacteria, Bacillus subtilis. In 1872, B subtilis became the first species known to produce endospores. It has been a laboratory favourite ever since. To keep things dry, each vial contains a silica bead, like you find with a new pair of running shoes. On the upper shelf in each box, vials are surrounded by a thin sheet of lead, partially shielding them from the harmful effects of background radiation.
Using this setup, scientists can monitor bacterial survival, and the effects of radiation on this, over timescales of decades and centuries. Every two years for the first 24, then every 25 years until the end of the experiment on 30 June 2514 (a Saturday), the box lids will be opened and a subset of vials from each shelf will be removed, analysed, and compared. The number of cells that germinate and those that don’t will provide a proxy of viability over 32 rounds of dried cyanobacteria vs multilayered endospore.
Is it worth maintaining some metabolic activity to repair the inevitable damage? Or do endospores represent the acme of longevity?
Using similar techniques to conservationists tracking the waning population of a threatened species, the experiment promises to answer elusive ecological questions about microbes. In their dormant state, for instance, do all cells die at the same rate? Or are there a stalwart few who live for an unusually long time? Alternatively, do most of the cells survive up to a certain point, after which population collapse is inevitable? The only way to make progress on these questions is to ‘quantify the mathematical function that describes their death’, Cockell says.
The experiment also promises to reveal the extent of molecular damage over time. By peeling away the bacteria’s outer shells, Cockell hopes to look at their DNA and other molecular innards, collating the accumulation of errors. Similar experiments have revealed around 60 double-strand breaks in bacterial DNA after just six weeks of desiccation. But what happens over six decades, and longer? Is it worthwhile to maintain a small amount of metabolic activity in order to repair the inevitable damage, as some bacteria from ice cores aged 500,000-years-plus suggest? Or do endospores represent the acme of longevity? How best to preserve that room?
Over the coming centuries, this is what Cockell’s experiment should be able to tell us. And even if it doesn’t reach its farfetched target of 500 years, each successive sample will provide a new insight into microbial dormancy, answering questions of life and death that have never been asked before.
By placing a surplus of samples in each box, Cockell has created an archive of information that other scientists can tap into. ‘There’s complete flexibility,’ he says. ‘The samples are just sitting there and people can do all sorts of experiments depending on what technology is available.’ Brian Wade from Michigan State University, for instance, saw a description of the ‘500-year Microbiology Experiment’ in the journal Astrobiology, and decided to ask Cockell if he could get involved. Wade, a member of Richard Lenski’s E coli long-term evolution experiment at Michigan, wants to address the question of how dormancy affects a microbe’s ability to adapt and evolve.
After the bacteria are revived in the UK, some will be preserved in glycerol, frozen and then shipped over to the US. ‘Each time point in Cockell’s experiment will essentially become a new ancestor for a new set of evolutionary lines,’ says Wade. Within each line, a subset of microbes will be allowed to multiply away from their ancestors for a certain number of generations before being frozen themselves, saved for later use like files on a hard drive.
By reviving cells from this ‘frozen fossil record’, Wade plans to make ancestors and descendants compete on nutrient-rich agar in a Petri dish. From the survivors, he can calculate how dormancy affected their capacity to adapt to new stressors – such as competitors. As each new sample (two years, four years, six years and so forth) gets sent to the lab, grown, and then thrown into competition with their ancestors, Wade predicts that the longer the period of dormancy, the lower the microbes’ capacity for de novo evolution. Will longer rests, and all the genetic damage that seem to go with them, really weaken the breed?
The micro-organisms will be dead, unable to germinate from their deep sleep even when given an Eden of food and water in a Petri dish
But simply by attempting this experiment, Cockell also highlights a common shortcoming of studies on bacterial dormancy: they’re anthropocentric, limited by grant cycles and human lifespans. ‘[Microbes] don’t recognise these barriers; they grow and desiccate over century and millennial timescales,’ says Cockell. ‘We have an experiment here that is more in line with the way microbes behave.’
In their suspended states of animation, microbes exist in a realm completely apart from most other organisms. They are neither living nor dead. Without growth, reproduction, and (in the case of endospores) metabolism, they lack many features that seem inherently tied to being alive on Earth. Indeed, they are just one borderline case that makes a sweeping definition for life nigh‑on impossible. Writing last year in The New York Times Magazine, Ferris Jabr commented that the problem comes about because scientists ‘have been trying to define something that never existed in the first place’. He concluded that ‘Life is a concept, not a reality.’
So what about death? We can assume that there will be a cessation of health over time until there is no point of return. There will be no anabiosis or reviviscence. The micro-organisms will be dead, unable to germinate from their deep sleep even when given an Eden of food and water in a Petri dish. This is what Cockell, his students, and their successors hope to define in bacteria using the 500-year experiment. It is not a topic of semantics, but a systematic evaluation of loss of life over time.
As of now, there are no results to report. Nor will there be until 2048, when the first study can be published. Cockell hopes to be around for this day. ‘But if not, there are students who can carry the experiment,’ he says. It’s a legacy, a dedication to microbes that carries with it an emblem of its inspiration. Within both of the boxes is a sealed Petri dish containing dried agar and Chroococcidiopsis cells. Each one is an embodiment of scientific curiosity. Cockell found that they could survive 10 years. What happens beyond this? Only time will tell.