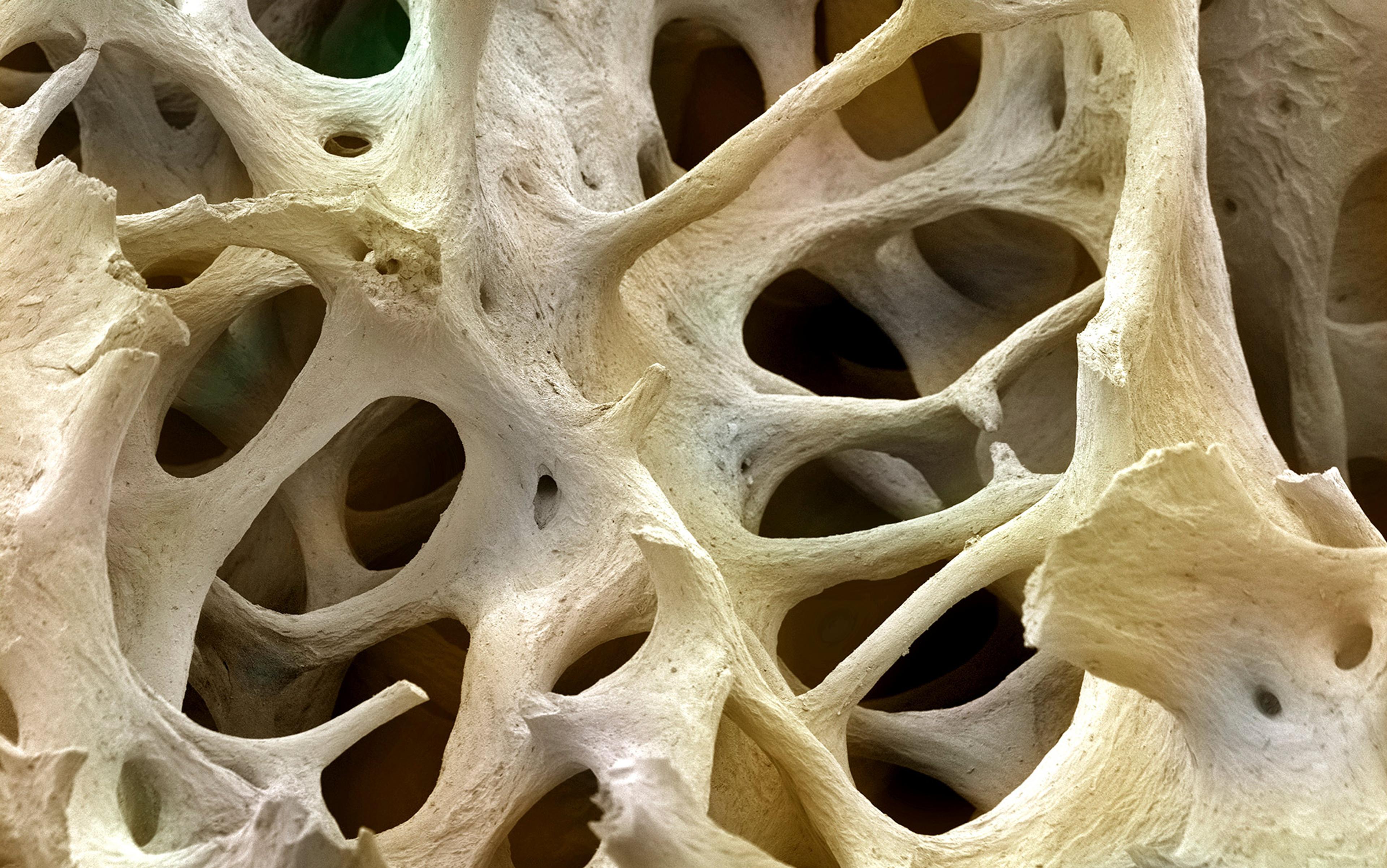
A little over 20 years ago, the physician and geneticist Gerard Karsenty thought that he knew the secret of bone mineralisation, the vital process by which calcium, phosphate and other minerals are bound to a matrix of proteins to make bone hard. There was a small protein, called osteocalcin, which bone cells made and released, and to which calcium avidly stuck. These properties convinced Karsenty that osteocalcin was the means by which bone cells controlled bone-hardening; and so, at his Columbia University lab in New York, he began engineering mice lacking the osteocalcin gene. He predicted that, without osteocalcin, their bones would be a fragile mess.
But that’s not what happened. Bone mineralisation was completely normal – losing osteocalcin had zero impact on this process. In fact, the bones of the engineered mice were heavier than before. The results felt like a disaster.
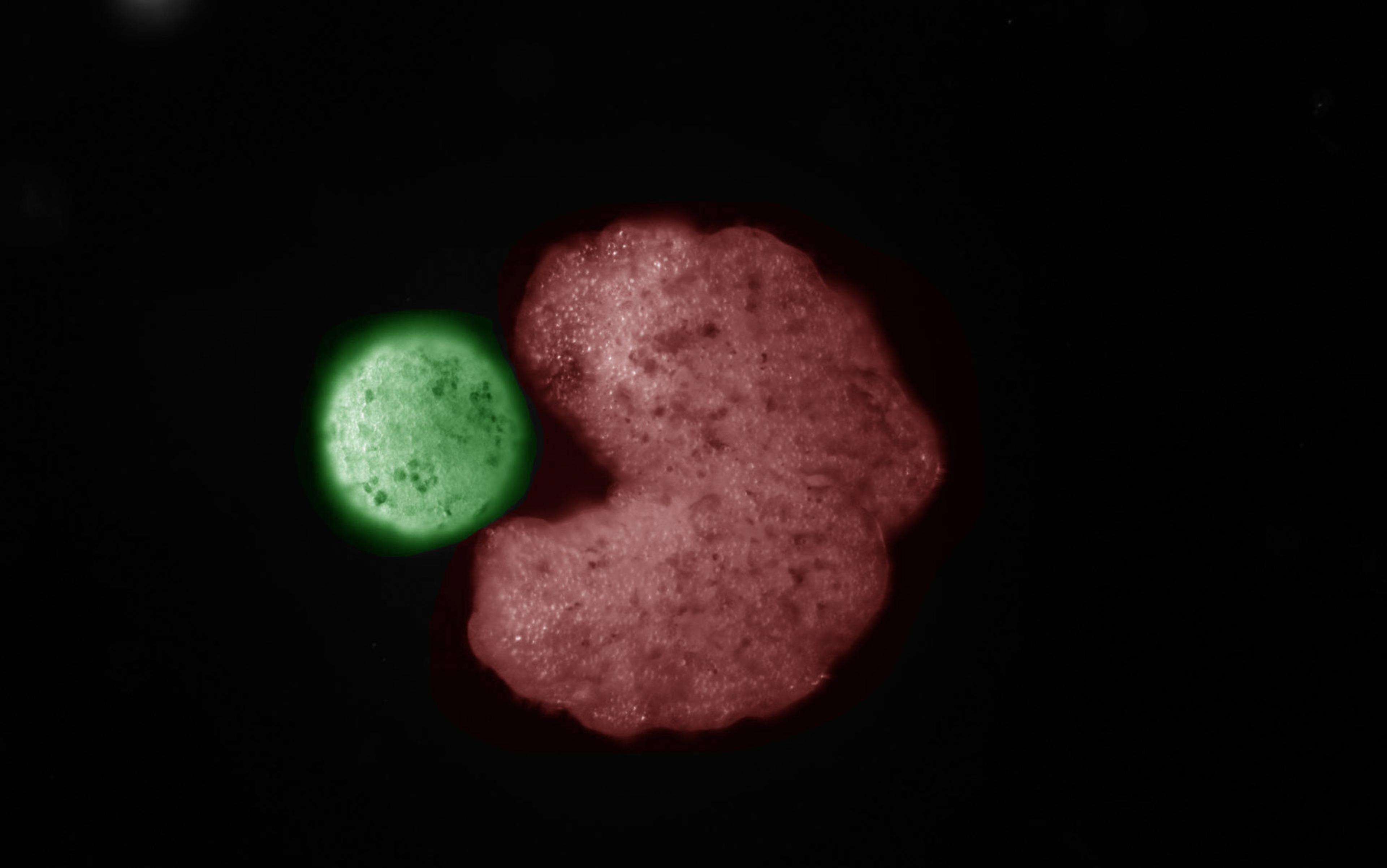
Then the postdoc who was examining the bones noticed something puzzling: when osteocalcin was absent, getting to the skeleton required her to dig through more fat tissue. It was a curious result that no one had expected. How could tinkering with bone have made the mice obese?
Karsenty thought that they might have mistakenly knocked out the wrong gene. If they’d accidentally removed a gene crucial for fat metabolism and left osteocalcin intact, this would account for both the fat and the normal bones. But no, the genetics had been done right.
There was just one other way to see it: osteocalcin, known to be present in healthy mice, not just in bone but also in blood, was not the agent responsible for bone mineralisation. Instead, it was moving from the bone into the blood, then travelling to fat tissue, where somehow it influenced how that tissue behaved. Because osteocalcin travelled via the circulation from a point of origin (in this case, bone) to a distant site (here, fat tissue) to exert a meaningful biological action, it was what scientists call a hormone.
Bone had long been appreciated as much more than an inert internal scaffolding – step by step, it had been shown to be the seat of red blood cell formation, the body’s dynamic reservoir of calcium, and a tissue that, uniquely in our bodies, is constantly, actively broken down then remade. But still, the idea that it was also an endocrine organ – the definition of any organ excreting hormones – and that it played an essential role in keeping mammals trim, seemed too much. ‘It took us 10 years to accept it,’ says Karsenty. ‘It took me 10 years to accept it.’
Karsenty and others eventually confirmed that bones secrete hormones essential for an animal’s health. And with that finding, the skeleton joined a growing list of tissues shown to participate in a body-wide conversation between organs. The traditional concept of the endocrine system as a second-command system working in tandem with the nervous system – and largely directed by the brain – is being replaced with a more autonomous view of interorgan communication, one in which most, if not all, organs have a voice. Grasping the logic of a control system in which the body’s organs are both the targets of hormonal commands and the source of them is still only beginning, but the clinical implications are sure to be profound.
From the mid-19th century onwards, a handful of physiologists started to speculate about the existence of chemical messengers that would act within the body. Most famously, when the French physiologist Claude Bernard proposed his ideas about how the body functions to always maintain a stable internal milieu, he speculated that ‘internal secretions’ were a key part of this regulation. As the century proceeded, the idea that certain tissues – such as the adrenal and thyroid glands, plus the gonads – released some sort of bioactive messengers was increasingly secure, owing in large part to clinical observations.
The first hormone was discovered at University College London on the afternoon of 16 January 1902. Ernest Starling and William Bayliss, collaborators and brothers-in-law, were testing a rather controversial idea. The great Russian physiologist Ivan Pavlov – he of the bells and salivating dogs – had declared that the release of alkaline digestive juices from the pancreas was controlled by the nervous system. But Starling and Bayliss had doubts, and instead speculated that it was the small intestine itself, the organ into which the juices flowed, that stimulated the pancreas. They hypothesised that acidic stomach contents descending into the intestine triggered the release of a blood-borne message that travelled to the pancreas, to activate it quite independently of the nervous system.
To test this, Starling and Bayliss worked with an anaesthetised dog, removing the nerve supply from a region of the animal’s small intestine but leaving the blood supply intact. They then stimulated the intestine’s insides with hydrochloric acid, and waited to see if pancreatic secretions were still triggered. When the digestive juices came forth, Starling declared: ‘Then it must be a chemical reflex.’
When a biological system is perturbed – a constant of life – it acts to minimise the extent of the perturbation
But he wasn’t done. He immediately ground up some of the intestinal lining with sand and more acid, then filtered the mix and injected it into the dog’s jugular vein. When Starling and Bayliss saw the pancreas release its contents even more profusely, they knew they’d isolated a messenger that could travel through the blood to act at a distant site. They called the chemical secretin.
What was uncovered was a negative feedback loop – a concept that would become increasingly central to endocrinology and physiology. The acidic material arriving from the stomach triggered the release of a messenger. That messenger then travelled to the pancreas to evoke the release of alkaline digestive juices to neutralise the intestinal contents, thereby terminating the release of the messenger. It is a specific instance of a general control mechanism. When a biological system is perturbed – a constant of life – it acts to minimise the extent of the perturbation. If blood sugar goes too high, the hormone insulin is released from the pancreas in response. That insulin instructs multiple tissues to absorb the excess glucose, reducing blood sugar levels. If you are cold, you move to a warm place or you shiver – behavioural and physiological methods, respectively for making you less cold.
Apparently, it was at a Cambridge dinner that a scholar of Ancient Greek overheard Starling explaining the notion of a chemical reflex to a colleague, and suggested that the travelling chemicals be called hormones after the Greek for ‘excite’ or ‘to stir up’.
Starling first used the new term publicly in his lecture series, ‘The Chemical Correlation of the Functions of the Body’ (1905). In these Croonian Lectures at the Royal Society in London, he pondered the evolution of hormones, suggesting that they appeared to be a very early event in animal evolution, with the earliest multicellular creatures relying entirely on diffuse chemical signalling. He also said that while evolving nervous systems had ultimately equipped animals with the means to quickly respond to their circumstances electrically, this had not replaced the network of chemical communications.
Starling divided his ‘chemical correlations’ into two types. The first type included those reactions triggered by an external event, for instance, the surge of adrenaline redirecting blood toward the muscles in the face of danger. The second type included reactions operating within the body no matter what was happening outside – for instance, the looping interactions of four primary hormones that regulate the female reproductive cycle.
Starling’s lectures, coming only two years after secretin’s discovery, feel strikingly prescient. He was certain that a great web of chemical signals passed between the body’s organs to mutually coordinate their activities.
From that point on, the number of known hormones started to accumulate. The biological actions of extracts from glands known to have endocrine functions were described, then whittled down to their active components. In the 1920s and ’30s, the sex hormones oestrogen, progesterone and testosterone were isolated and characterised. In the 1950s, hormones released from the brain – from the pituitary gland, and the hypothalamus – were described, and their interactions with glands making hormones elsewhere were charted. After that, the ways that hormones influenced their target cells at a molecular level were revealed – some acted briefly, others had longer-term effects by changing gene expression.
The concept of target tissues was important. There were the endocrine organs – most of them glandular tissues, whose primary function was evidently to make and secrete things – and there were the organs on which hormones acted. Textbook depictions showed, and often still do, the endocrine system as a handful of glands plus the gonads: an archipelago of hormone-making islands dotted about the body.
But the list of hormones never stopped growing. And neither did the list of tissues that made them. Certain hormones long known to regulate blood flow, gut absorption and blood-cell production turned out to be coming from the kidney. The liver was shown to make and dispatch hormones. And secretin became only one of a number of circulating signals emanating from the digestive system.
What were once known only as target tissues are, we now know, doing the shooting
In the 1970s, the importance of two-way interaction between the brain and the immune system was etched in stone. Then, in the early 1990s, when scientists at the Rockefeller University in New York sought to learn why a well-known strain of laboratory mice were morbidly obese, they discovered that it was because the mice lacked a hormone. Christened leptin, this hormone potently suppressed appetite, and was made and released by white fat tissue. Leptin’s ability to make mice eat less sparked massive interest in somehow harnessing its biology to create drugs that did likewise for humans – a goal that remains still, for a variety reasons, unfilled.
But as the quest for an appetite-quenching drug gathered pace by the turn of the millennium, Karsenty was looking at other functions of leptin. The hormone, he learned, also regulated bone mass – an early confirmation that energy storage and its use were intimately linked to bone. At the same time, other researchers were making a case for skin as an endocrine organ, and gaining a fuller understanding of signalling molecules released by the heart.
In aggregate, these threads tell us that most organs have an endocrine function. What were once known only as target tissues are, we now know, doing the shooting. If an organ is not known to secrete a significant hormone, it seems reasonable to ask if people have looked hard enough. Well over a century after Starling gave his Croonian Lectures, it appears that biology is finally close to realising the full extent of his ‘great system of correlations’.
The breadth of tissues now known to release hormones demands that we rethink the very idea of the endocrine system itself. If it isn’t a restricted set of glandular tissues that direct the behaviour of the body’s various organs – a control system complementary to the nervous system – how should we conceptualise it instead? It appears to be something far more pervasive and democratic – a system through which all the body’s organs broadcast their status by discharging molecules into the blood and so, together, shape what the body is doing at any given time.
Some support for the idea came from the discovery of a different molecule made and released by bone that was quickly recognised as a hormone. Discovered first in a cancer that caused bone wasting, the molecule, christened FGF-23, ensured the amount of phosphate available matched the amount the skeleton needed, establishing that bone made a hormone. But Karsenty doesn’t think it helped establish bone as a significant endocrine organ. “FGF23 regulates a function that is intimately linked to bone health,” he says. It seemed to resemble secretin: a hormone that functioned in a self-contained loop allowing a single organ to better do its job.
It was in 2007 that Karsenty nailed the bigger idea through osteocalcin; to explain why mice lacking it were fat, bone had to be an endocrine organ that wasn’t merely regulating its own biology. Researchers had already shown that fat cast a vote in how the body handled energy resources by making hormones that regulated appetite. Now bone, too, entered an expanding network of organs that together worked to maintain energy homeostasis.
Then, Karsenty’s group expanded the network of interactions in a paper entitled ‘Endocrine Regulation of Male Fertility by the Skeleton’ (2011). In this study, they described how male mice lacking osteocalcin didn’t breed as successfully as males with the hormone, showing a role for the molecule in modulating testosterone synthesis as well.
These two studies showed that bone spoke back to organs known to regulate it. Hormones from both fat tissue and the gonads act on bone, and suddenly there was evidence that rather than these organs simply directing bone, the tissues were engaged in a conversation. Karsenty predicts that it will be a general principle of endocrinology ‘that there is no one-way street between one organ and another’.
But the story wasn’t finished yet. There was another characteristic of the osteocalcin-knockout mice that needed exploring – their behaviour. People in the lab had variously reported that the mice appeared either dim or abnormally quiet. And it was time to scientifically dissect this.
The fact that bone modulated neural function meant the brain also had to be welcomed into the fold
Through a series of careful experiments, Karsenty’s post-doc Franck Oury and other team members found that the brain contains a unique receptor for osteocalcin; through its actions at this neural receptor, osteocalcin was modulating brain function. Mice lacking the hormone have appalling memory skills, live in an apparent state of anxiety, and have seriously altered levels of various neurotransmitters.
The initial work had yielded a framework for mutual dependencies between skeletal, metabolic and reproductive tissues. The schema made intuitive sense: energy resources needed to be appropriately allocated to maintaining bone health and for mating. But the fact that bone modulated neural function meant that the brain also had to be welcomed into the fold. ‘We have to try to understand the evolutionary logic to having a molecule and an organ controlling memory, running and reproduction,’ says Karsenty. ‘And that is what we are working on now.’
The evidence keeps coming. One paper published in November 2018, explored a broader role for the bone hormone, FGF-23, beyond just handling phosphate. Another paper, out that same month, described new functions of the original hormone, secretin: in addition to its actions on the pancreas, secretin also stimulates brown fat tissue after a meal. Activated brown fat tissue generates heat, raising body temperature and inhibiting food intake.
The geneticist Norbert Perrimon works with the fruit fly Drosophila at Harvard Medical School. In the past, he’s developed the sorts of genetic tools that make this organism such a wonderful creature for the study of biology. But recently, he’s started to ask how the animal’s tissues sense various internal conditions, and what messages they release beyond their own borders. Or, using the term he coined with his graduate student Ilia Droujinine in 2013, he now studies the interorgan communication network (ICN). More than the older term, endocrinology, the acronym describes how the field is evolving now. ‘I think, as soon as you touch one organ, it’s going to elicit a response in other organs,’ Perrimon says.
One of the lab’s early studies involved factors, called myokines, excreted by muscles to modulate growth and energy distribution. The Perrimon team was especially interested in myokines and ageing. Using a genetic trick, the lab’s Fabio Demontis selectively decreased the ageing of certain flies’ muscles. Then he looked across the whole insect to see if ageing had also slowed in other tissues. It had – a result suggesting, remarkably, that the animal’s overall ageing was regulated by factors that the muscles released into the Drosophila haemolymph, the flies’ equivalent of blood.
The wider implication was that muscle is prone to wear out early in flies – and potentially in mammals, too – perhaps due to the harsh demands of a life spent constantly contracting and relaxing. When the myokines released by healthy muscles fall away, the whole animal declines. Since the 2010 publication of this study, Demontis has built his career on understanding exactly which myokines are involved and whether they might be harnessed to treat age-related decline in humans. (Indeed, recent years have seen a surge of interest in the idea that changes in circulating factors are elemental to the ageing process. This has been driven, in no small part, by experiments showing that blood from young mice can rejuvenate numerous tissues, including the brain, of older animals. Results from Karsenty’s group suggest that replenishing osteocalcin can have a key role in these effects.)
They drew arrows to show newly identified hormonal links. The result was a frantic mishmash of connections
The myokine work is fairly representative of the Harvard group’s initial effort to discover hormonal factors arising from one tissue to influence others. But now Perrimon wants to be more systematic about defining the entire ICN. To do so, his lab is employing a simple but elegant screen. It involves genetically introducing an enzyme into one organ at a time, thus tagging all the proteins that this organ secretes. This widely used tag was chosen because there’s a second molecule that sticks to it – and only it – like glue. The researchers collect all the fly’s other organs, one by one, to generate lists of all the proteins that organ X releases to end up in organ Y.
Much as with mammals whose muscles and fat release hundreds of different proteins, these screens have identified comparable numbers in fruit flies. The lists include tens of previously unknown proteins. Perrimon is focusing on those conserved from flies to mammals.
‘I believe there are going to be many, many new things that we can discover soon,’ he says, though ‘what’s really time-consuming is the follow-up analysis to demonstrate that the factors are really doing interesting things.’
When a new hormone is identified and shown to have a significant function, it buys itself a line on a diagram like the one Perrimon and Droujinine constructed for a 2016 review detailing their plans to characterise the ICN in fruit flies. Starting with the recent finds in mammals, they arranged, roughly in a circle, skeletal muscle, pancreas, brain, bone, gut, testes, heart, kidney, fat tissue and liver. And then, they drew arrows to show newly identified hormonal links between tissue pairs. The result was a frantic mishmash of connections.
The classical notion of individual negative-feedback loops – each keeping a single physiological variable in check – remains a vital concept in biology. But it’s now clear that there are many loops and that they are all interlocked.
Perrimon is now determined to make greater sense of the new diagrams. ‘We’re looking at fat to gut, gut to muscle, muscles to fat, muscles to gut,’ he says. He pauses, then says with a laugh: ‘And it’s getting very confusing.’
So why do most, and potentially all, tissues have an endocrine element to them? Echoing Starling’s early thoughts, Perrimon and Droujinine have written that it might be as fundamental as going back to the advent of early multicellular life. As organisms became more complex, and different types of cells evolved to have specialised functions within a cellular collective, there was a need to coordinate those functions. As an example from later in evolution, when muscles began driving movement, they likely relied on fat cells for nutrients. While one can see that a nervous system signalling to both tissues could make chemical communication redundant, it’s now clear that the hormonal link – and the autonomous character of peripheral tissues – never went away. As specialised populations of cells evolved, the total organism benefitted from individual tissues broadcasting their status to modulate other organs. As Karsenty puts it, ‘no organ is an island in our body.’
A critical event occurred, says Karsenty, when vertebrates moved from water to land. When bony fish left their aquatic homes around 390-360 million years ago, the skeleton had to become a much more substantial structure to allow movement over land – gone was the buoyancy of water, and gravity had to be resisted by robust bones. These expanding bones increasingly demanded energy. And no longer were terrestrial animals free to exchange calcium and phosphate with their aquatic surroundings. Instead, these minerals had to be eaten, stored and regulated. Bone helped solve this problem by becoming a dynamic reservoir for calcium storage and release.
Karsenty’s occasional collaborator Douglas DiGirolamo has written that these new or dramatically extended bone functions couldn’t evolve in a vacuum. Rather, a mineralised skeleton had to integrate itself into existing endocrine loops by responding to hormones and by releasing them. In this way, bone made the loops more layered and complex, and carved out a new niche for itself in the animal’s physiology.
Another organ that does this, not over evolutionary time but again and again in the course of a mammalian pregnancy, is the placenta. Once it has accessed the mother’s bloodstream, a placenta secretes a wealth of hormones that reshape maternal physiology to support the foetus’s needs. Mother and offspring might be seen as a single network of hormonal interactions, rather than two separate networks abutting one another.
‘We need to break open the boundaries between these silos, organ by organ’
That the placenta contains the genes of the foetus’s father can lead to it trying to game the mother’s physiology in favour of the foetus. But this type of gaming has a much darker character in another system that Perrimon is studying extensively: a type of gut tumour, introduced into fruit flies, that appears to use a suite of hormones to shift the body toward conditions that the tumour needs to thrive. The cancer cells liberate sugar, causing other tissue to waste so that the tumour can feed itself. It is, Perrimon hopes, a model that will offer helpful, fresh perspectives on the process of muscle wasting in human cancer patients.
These kinds of insights can lead to improved treatments and care for cancer and metabolic disease. ‘By looking at one organ at a time,’ says Karsenty, ‘we may miss interactions between organs that are functionally extremely important. And if they are functionally extremely important, they are de facto medically extremely important. We need to break open the boundaries between these silos, organ by organ.’
But on an academic level, the sheer complexity of the ICN is what troubles Perrimon. What’s currently most perplexing, he says, is that certain hormones have different, even opposite effects according to the conditions under which they’re released, and according to which tissue has released them. If factor Z is released by muscle, for instance, it can have a different impact on the brain than when fat secretes factor Z. Maybe this is because factor Z reaches the brain accompanied by other factors, depending on circumstances, and these together determine how the brain responds. Or perhaps the brain is already in a different state when fat releases factor Z, compared to when muscle does. The conundrum prevails wherever signalling molecules are from, be it fat, muscle, the gut or immune cells.
If the ICN, or the endocrine system, is an instructive set of commands, its internal chemical logic needs to be determined. Unlike the nervous system – where neurons make physical contact with the cells they influence – there is no anatomical specificity to the endocrine system. Cells simply secrete molecules into the passing circulatory fluid, and those molecules travel off to encounter whatever they might encounter. All that exists at a given point in time is a volume of blood representing the state of all the body’s diverse and dispersed tissues. Given the mind-boggling complexity, Perrimon worries that current experiments typically track only a few variables – such as the hormone of interest and one or two outcomes of interest. If many systems are interacting, the many systems need monitoring.
Considering what’s been achieved so far, he says: ‘The studies are fine, they are telling something about what those factors are doing, but we’re only getting one little piece of the puzzle. And what we don’t understand right now, is the complexity of that puzzle.’