In March 2019, we planted a garden full of monsters outside the Biodesign Institute at Arizona State University (ASU). Their limbs rise out of the ground, reaching in every direction, doubling back on themselves as they expand and grow. These beautiful creatures are crested cacti – plants with mutations that lead them to have gloriously wild growth patterns that have many parallels with cancer.
We wanted to create an opportunity for people to come together around cancer in a way that was positive and hopeful, while acknowledging the often-deadly realities of the illness. Like cancer in humans, the out-of-control growth seen in these cacti, which causes their tips to fan out into glorious crests or sometimes creepy brain-like patterns, arises from disruptions in cellular behaviour, often as a result of mutations.
Also paralleling cancer in humans, these growths – technically known as fasciations – can make the plants more vulnerable to disease and death by weakening them. Yet crested cacti often live with their cancer-like growths for decades. And, because of their unique sculptural beauty, crested cacti are highly sought-after. Botanists and gardeners tend to these rare specimens with great care, helping them to survive and thrive despite their vulnerabilities.

Crested cacti show us that we are not alone. Cancer, and cancer-like growths, are a problem for all forms of multicellular life – from coral to cacti and from mice to elephants – and have been since multicellularity first evolved around 2 billion years ago. This is because cancer is inexorably tied to the cooperative nature of multicellularity, which allows groups of cells to accomplish collective goals that are impossible for cells operating only as individuals.
Multicellular life evolved many systems that establish and enforce cellular cooperation – including controls on cellular proliferation, resource use and cell death – to ensure that no particular cell would take advantage of the cooperative multicellular group. But these cooperation-enforcement systems aren’t perfect. Sometimes free-riding cells manage to escape these controls and exploit the cooperation inherent in the multicellular body. When this happens, it results in what we know as cancer.
Cancer cells thrive and proliferate within the body because they take more resources and opportunities for themselves, and don’t follow the rules of multicellular living that most healthy cells do. They also fail to undergo programmed cell death if they become a threat to the multicellular organism (for example, when they have too many mutations). This cheating gives cancer cells a competitive advantage compared with healthy cells, allowing them to expand in the population and threaten the viability of the organism.
You can think of this as a type of evolution playing out within the body, but on a microscopically small scale. Cheaters – cells that proliferate too much or use resources too quickly – will thrive and become more frequent in the next generation, evolving to get progressively more effective at exploiting the multicellular body. This means that cancer will potentially be a problem for any multicellular life form, including humans. (With about 30 trillion cells constantly cooperating and coordinating their behaviour and gene expression, we are bastions of the multicellular strategy.)
Crested cacti can help us see our susceptibility to cancer, not just in terms of the evolution of organisms during the history of life on Earth, but as a result of evolutionary processes happening within the body during the lifetime of an individual. Cells evolve within multicellular bodies through a process called somatic evolution (after ‘soma’, meaning body). Somatic evolution is just like organismal evolution that happens in the natural world, except in somatic evolution natural selection is acting on the cells inside an organism, rather than on whole organisms.
If you were to sketch out the evolutionary tree of somatic cells in the body (akin to a family tree that depicts an ancestral lineage), an occurrence of cancer would look like a particularly bushy branch in that tree – cancer cells proliferate rapidly because they fail to die when they should, and they greedily consume the body’s resources. The crests that form as crested cacti grow show the ‘bushiness’ of the evolutionary tree in a literal, embodied way. Whereas looking with the naked eye at a tumour in a human or nonhuman animal doesn’t reveal much about its history or evolutionary structure, with crested cacti you can see the evolutionary history and trajectory of the mutated cells in the branches that grow and expand in all directions.
The visual embodiment of the runaway growths on these cacti makes it possible for us to viscerally understand something profound about cancer, without a microscope or complex statistical genomics. We can see how cancer is literally an outgrowth of the organism itself, rather than something ‘other’. It’s connected to the rest of the organism through its history and biology. The crests arise when cells in the meristem (the plant equivalent of human stem cells that have yet to differentiate into specialist cells) form a band, rather than a point as they do during more typical plant development. As the cells in the meristem replicate, they create rippling bands of proliferating plant tissue (rather than a singular growing tip, as is more typical among other plants).
Cheater detectors monitor the cell for any signs of ‘misbehaviour’
Thankfully, multicellular life has evolved ways to keep cancer under control, and crested cacti embody this too. When plants such as cacti develop cancer-like forms, they typically don’t spread throughout the plant, making it easier for the plants to survive and thrive despite the growths. Similarly, across all multicellular life, cancer suppression systems have evolved – for example there is a tumour suppressor gene TP53, found in humans and other animals, which encodes the protein p53 that monitors cells for DNA damage and initiates a process of cellular suicide if the damage can’t be repaired.
TP53 and other cancer suppression systems help to make sense of why bigger animals aren’t more prone to cancer, a phenomenon known as Peto’s Paradox (named after the English statistician who first noticed it). Logic would suggest that, because they have more cells for a longer time, particularly large and long-lived animals would experience a higher incidence of cancer than smaller, shorter-lived species, yet that is not the case. Elephants, for example, have more cells than any other land animal, but they evolved multiple copies of TP53, which likely explains why they aren’t more vulnerable to cancer.

You can think about genes such as TP53 as the cheater detectors of the genome: they monitor the cell for any signs of ‘misbehaviour’ and then either correct it (through DNA repair) before the cell can replicate or, if necessary, initiate cellular suicide to protect the whole organism. TP53 is just one of many cancer suppression systems that multicellular life forms have evolved. Humans have two copies of TP53: one inherited from our mothers, one from our fathers. When one of these copies is mutated – as occurs in the heritable disease Li-Fraumeni (named after the two American physicians who discovered it) – lifetime cancer risk skyrockets.
The largest mammals on Earth, whales, appear to have especially intense cancer suppression systems. Sequencing of the bowhead whale genome revealed duplication of the gene encoding PCNA, a protein that is crucially involved in accurate DNA replication and repair. Our team at the Arizona Cancer Evolution Center has looked at the humpback whale genome, and the genomes of other whales as well. We’ve found evidence that they’ve evolved a variety of tumour suppressor genes, including genes encoding for DNA repair and suppression of metastasis (the spread of cancerous cells from one part of the body to another).
The day we opened the cancer garden, and at subsequent public events, we set up a table where people could learn more about the cacti, talk with researchers and have the opportunity to write, on silver tags, the names of people who have been affected by cancer. ‘It’s nice to have your experience documented and not your death,’ says Mindy Miller, a cancer-patient advocate on our team who has also become a personal friend of mine. Mindy and another of our patient advocates, Chevas Samuels, have since spoken with countless visitors to the garden as they fill out these silver tags and hang them. ‘I’ve had some really happy, sad and bittersweet conversations with people who stop at our tables to add names to the garden,’ Chevas tells me.

‘So many lives are affected because every person living with cancer is a mother, brother, father, sister, friend, colleague, acquaintance, and we are all tied together,’ Mindy adds. ‘What happens to one affects all.’
The most profound and heartening lesson that crested cacti can teach us is that they and we – as multicellular life forms – can sometimes live with cancer. Multicellular life has grappled with cancer since its very beginnings, and has evolved to keep it under control. By looking to crested cacti and how they live with cancer – and also looking more broadly across the tree of life at how organisms suppress cancer – we can discover new and more effective ways of preventing and treating the illness.
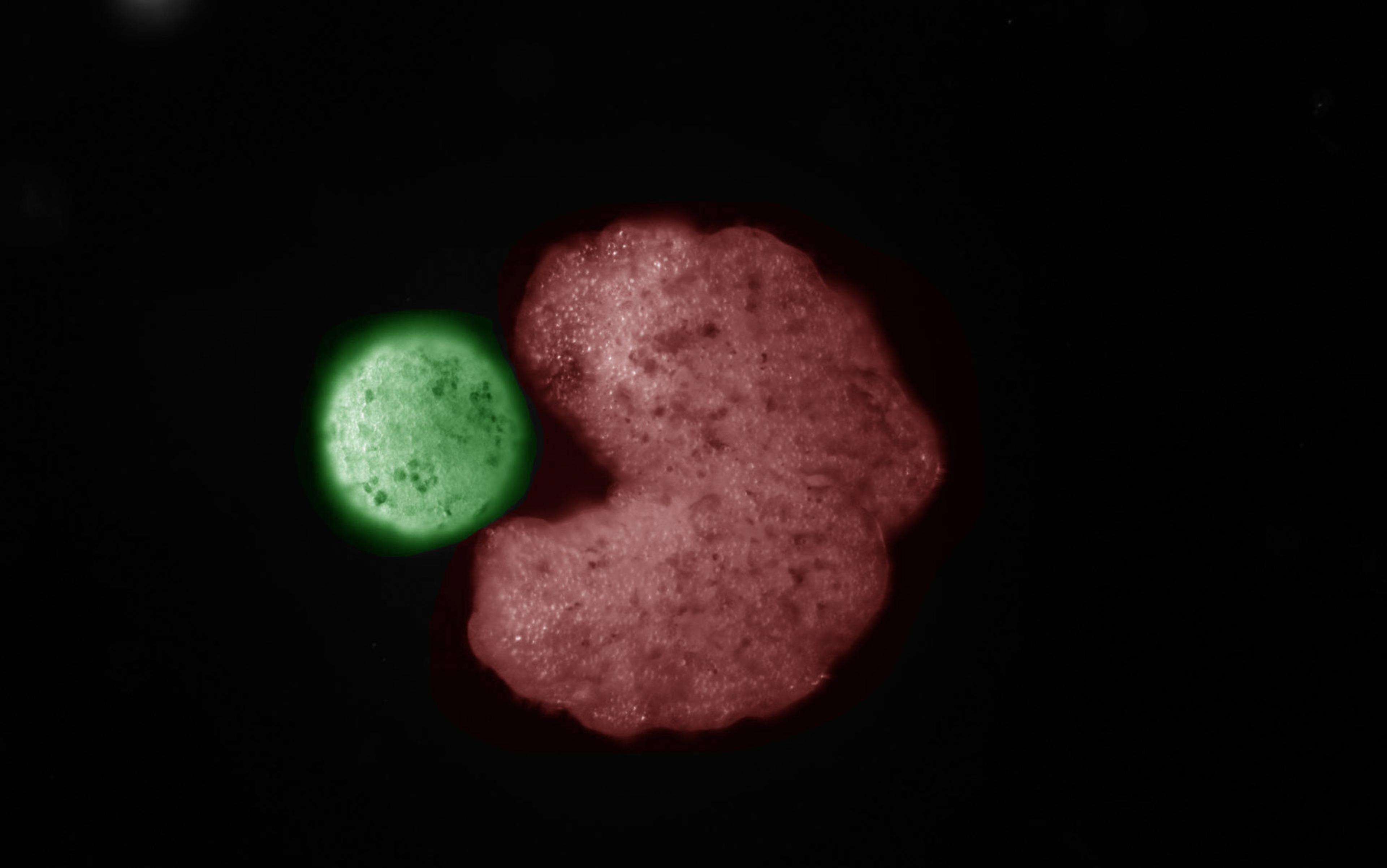
One novel, radical approach is called adaptive therapy, which aims to control the tumour – by keeping it at a stable size – rather than eradicate it, which is the conventional treatment approach. Adaptive therapy is one of the most successful treatments so far to emerge from an evolutionary approach to cancer. Pioneered by Robert Gatenby, an oncologist and evolutionary biologist at Moffitt Cancer Center in Florida, it was first tested in mice a decade ago, and is now in clinical trials with patients. First, a tumour is treated to bring it down to a more manageable size, after which it’s treated further only if and when it grows above a certain threshold. Depending on the trial in question, there are variations in the exact algorithm used to make this judgment, but all adaptive therapy trials share the general principle of treating the tumour with the goal of control rather than eradication.
Adaptive therapy was successful in controlling tumours in mice, and the first human trials have been promising
One critical problem with traditional chemotherapies is that the rapid high doses – which are aimed at eradicating the tumour – can actually end up selecting for cancer cells that are resistant to the drugs. When the cancer grows back (as it often does), the drugs no longer work because all of the cells that remain are ones that grew back from the few resistant cells that survived the high-dose therapy. Ironically, the higher the chemotherapy dose, the stronger the selection pressure favouring drug-resistant cells (because the differential fitness between sensitive and resistant cells is higher with stronger treatment).
Adaptive therapy and other approaches aimed at tumour control can sidestep this problem. With adaptive therapy, the tumour is never treated with the goal of eliminating it, only keeping it a manageable size. This means that some sensitive cells survive therapy as well, and so when the chemotherapy is removed, the tumour that grows back includes both sensitive and resistant cells. In fact, because there is typically some sort of cost to drug resistance (such as having pumps on the cell membranes that use a lot of energy to pump out the drug from the inside of the cell), in the absence of therapy, it’s often sensitive cells that grow better than resistant cells. When the drug is removed, sensitive cells can start to overtake the resistant ones, and as the tumour grows, it develops into a more manageable monster rather than a more dangerous one.
Adaptive therapy was successful in controlling tumours in mice, and the first human trials have been promising. In one, adaptive therapy kept prostate cancer patients’ tumours under control for 10 of the 11 patients involved – and they survived much longer than patients who received traditional treatment. The researchers have continued treating the patients in this trial, and Gatenby tells me they are seeing that the results are ‘better than the original report’.
Cancer is a terrible illness, but it’s also an intrinsic part of multicellular life. Keeping it under control for longer is a more realistic goal for the future of cancer therapy than hoping for a magic bullet that will eliminate cancer once and for all. Today, adaptive therapy is gaining traction, despite its main premise being contrary to traditional approaches. I’m working with colleagues at ASU and the Mayo Clinic to open an adaptive therapy trial for breast cancer after receiving funding from the Arizona Department of Health Services. Many other researchers are searching for funding to open their own adaptive therapy trials, and Gatenby and his colleagues at Moffitt are continuing to push forward with their work. They are accruing patients for a second prostate cancer clinical trial and, so far, no patient on adaptive therapy has seen their cancer progress. Such successful clinical trials are an essential step on the pathway to bringing adaptive therapy to the clinic.
Crested cacti show us the promise of the adaptive therapy approach. They embody the idea that having cancer and cancer-like forms can lead to greater vulnerability (like the greater susceptibility to infection that crested cacti have), but with tender care it is possible to survive and thrive, nevertheless. Also, there’s a monstrous beauty to their growths that, like cancer, are a manifestation of the creativity and resilience of life, even though their cellular success can compromise us as multicellular organisms. The question becomes, how to keep cancer under control – perhaps even allowing it certain aspects of its monstrous nature – while staying focused on keeping patients alive and their quality of life higher.
In the ASU garden, the little silvery tags hang from the trees shading these beautiful, mutated cacti. ‘Having a place to name those who are impacted feels like a quiet way of saying “I see you and I know you have struggled,”’ Mindy wrote when I asked her what the garden means to her. ‘The garden itself is a hopeful space … a place with a possibility that I wish for, both for myself and for future cancer patients.’
Cancer is part of who we are as humans and as multicellular life forms
Mindy’s words reflect the hopes that my colleagues and I at the Arizona Cancer Evolution Center have for the future of cancer. What if we think about caring for cancer patients as being less like helping them to eradicate pests and more like tending a garden of unusual and beautiful life forms? What if we approach cancer patients more like we approach crested cacti, giving attention to the extra care and management that they require in order to thrive? The garden of beautiful monsters we planted at ASU was inspired by the wild beauty of crested cacti and the parallels with human cancer. Our aim is to raise awareness about cancer’s evolutionary nature and to challenge the traditional way it’s treated.
Although it’s the first, we hope this won’t be the last garden of its kind. We’re working with hospitals and other research institutions to develop gardens that feature crested cacti and other fasciated plants. Hopefully, this will start more conversations among cancer researchers and clinicians about how we treat cancer and what we might do differently, but also create a space for cancer patients and their loved ones to experience cancer differently, as a part of life rather than just as a threat to it – and perhaps to catch a glimpse of the monstrous beauty that these cacti possess as they struggle with a fundamental challenge that life has faced since the dawn of multicellularity, the very same struggle that had to be overcome for multicellularity to evolve in the first place: keeping cellular cheating under control so that multicellular cooperation can thrive.
We decided to call our garden ‘Endless Forms Most Beautiful’. The phrase comes from the ending of Charles Darwin’s book On the Origin of Species (1859). ‘There is grandeur in this view of life,’ he wrote, ‘from so simple a beginning endless forms most beautiful and most wonderful have been, and are being, evolved.’ Cancer, in its many manifestations, is one of these endless forms that contribute to the grandeur of life. Despite the threats it poses to us, cancer is part of who we are as humans and as multicellular life forms. Our garden represents the continuation of this evolutionary story, providing a place of hope and transformation, encouraging us all to revise our conceptions about cancer and to continually adapt our approach to treating and managing it.